Hepatic parenchymal changes following transcatheter embolization and chemoembolization in a rabbit tumor model
- PMID: 23967098
- PMCID: PMC3743795
- DOI: 10.1371/journal.pone.0070757
Hepatic parenchymal changes following transcatheter embolization and chemoembolization in a rabbit tumor model
Abstract
Objective: To compare the effects of transcatheter arterial chemoembolization (TACE) with transcatheter arterial embolization (TAE) on liver function, hepatic damage, and hepatic fibrogenesis in a rabbit tumor model.
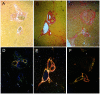
Materials and methods: Thirty-nine New Zealand white rabbits implanted with VX2 tumors in the left liver lobes were randomly divided into three groups: TAE, TACE, and control group. In the TAE group (n = 15), polyvinyl alcohol particles (PVAs) were used for left hepatic artery embolization. In the TACE group (n = 15), the tumors were treated with left hepatic arterial infusions of a suspension of 10-hydroxycamptothecin and lipiodol, followed by embolization with PVAs. In the control group (n = 9), the animals received sham treatment with distilled water. Serum and liver samples were collected at 6 hours, 3 days and 7 days after treatment. Liver damage was measured using a liver function test and histological analyses. Liver fibrogenesis and hepatic stellate cell (HSC) activation were evaluated using Sirius Red and anti-alpha-smooth muscle actin (α-SMA) immunohistochemical stains.
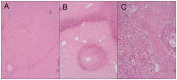
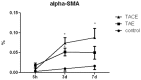
Results: TACE caused liver injury with greater increases in serum alanine aminotransferase and aspartate aminotransferase levels on day 3 (P<0.05). Histological analyses revealed increased hepatic necrosis in adjacent non-tumorous liver tissue from day 3 compared to the TAE group (Suzuki score of 2.33±1.29 versus 1.13±1.18, P = 0.001). HSC activation and proliferation were significantly increased in the TACE group compared to the control group at 3 and 7 days after treatment (0.074±0.014 vs. 0.010±0.006, and 0.088±0.023 vs. 0.017±0.009, P<0.05). Sirius Red staining demonstrated a statistically significant increase in collagen deposition in the livers in the TACE group 7 days after embolization compared to the control group (0.118±0.012 vs. 0.060±0.017, P = 0.05).
Conclusion: The results of this animal study revealed that TACE induced prominent hepatocellular damage and hepatic fibrogenesis, which compromised liver function and may be responsible for chronic liver decompensation.
Conflict of interest statement
Figures



References
-
- Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL (2002) A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer 94: 1747–1752. - PubMed
-
- Farinati F, De Maria N, Marafin C, Herszènyi L, Del Prato S, et al. (1996) Unresectable hepatocellular carcinoma in cirrhosis: survival, prognostic factors, and unexpected side effects after transcatheter arterial chemoembolization. Dig Dis Sci 41: 2332–2339.3. - PubMed
-
- Khan KN, Nakata K, Kusumoto Y, Shima M, Ishii N, et al. (1991) Evaluation of nontumorous tissue damage by transcatheter arterial embolization for hepatocellular carcinoma. Cancer Res 51: 5667–5671. - PubMed
-
- Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, et al. (2000) Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology 215: 123–128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

