Structure, antimicrobial activities and mode of interaction with membranes of novel [corrected] phylloseptins from the painted-belly leaf frog, Phyllomedusa sauvagii
- PMID: 23967105
- PMCID: PMC3742671
- DOI: 10.1371/journal.pone.0070782
Structure, antimicrobial activities and mode of interaction with membranes of novel [corrected] phylloseptins from the painted-belly leaf frog, Phyllomedusa sauvagii
Erratum in
- PLoS One. 2013;8(8). doi: 10.1371/annotation/dbb3e614-dc4c-40dd-b9e0-37787ae6b150
Abstract
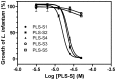
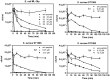
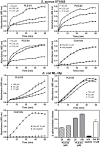
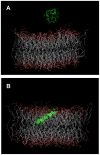
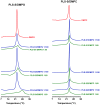
Transcriptomic and peptidomic analysis of skin secretions from the Painted-belly leaf frog Phyllomedusa sauvagii led to the identification of 5 novel phylloseptins (PLS-S2 to -S6) and also of phylloseptin-1 (PSN-1, here renamed PLS-S1), the only member of this family previously isolated in this frog. Synthesis and characterization of these phylloseptins revealed differences in their antimicrobial activities. PLS-S1, -S2, and -S4 (79-95% amino acid sequence identity; net charge = +2) were highly potent and cidal against Gram-positive bacteria, including multidrug resistant S. aureus strains, and killed the promastigote stage of Leishmania infantum, L. braziliensis and L. major. By contrast, PLS-S3 (95% amino acid identity with PLS-S2; net charge = +1) and -S5 (net charge = +2) were found to be almost inactive against bacteria and protozoa. PLS-S6 was not studied as this peptide was closely related to PLS-S1. Differential scanning calorimetry on anionic and zwitterionic multilamellar vesicles combined with circular dichroism spectroscopy and membrane permeabilization assays on bacterial cells indicated that PLS-S1, -S2, and -S4 are structured in an amphipathic α-helix that disrupts the acyl chain packing of anionic lipid bilayers. As a result, regions of two coexisting phases could be formed, one phase rich in peptide and the other lipid-rich. After reaching a threshold peptide concentration, the disruption of lipid packing within the bilayer may lead to local cracks and disintegration of the microbial membrane. Differences in the net charge, α-helical folding propensity, and/or degree of amphipathicity between PLS-S1, -S2 and -S4, and between PLS-S3 and -S5 appear to be responsible for their marked differences in their antimicrobial activities. In addition to the detailed characterization of novel phylloseptins from P. sauvagii, our study provides additional data on the previously isolated PLS-S1 and on the mechanism of action of phylloseptins.
Conflict of interest statement
Figures



Similar articles
-
Mechanism of antibacterial action of dermaseptin B2: interplay between helix-hinge-helix structure and membrane curvature strain.Biochemistry. 2009 Jan 20;48(2):313-27. doi: 10.1021/bi802025a. Biochemistry. 2009. PMID: 19113844
-
Discovery of Phylloseptins that Defense against Gram-Positive Bacteria and Inhibit the Proliferation of the Non-Small Cell Lung Cancer Cell Line, from the Skin Secretions of Phyllomedusa Frogs.Molecules. 2017 Aug 29;22(9):1428. doi: 10.3390/molecules22091428. Molecules. 2017. PMID: 28850103 Free PMC article.
-
Identification and Characterisation of the Antimicrobial Peptide, Phylloseptin-PT, from the Skin Secretion of Phyllomedusa tarsius, and Comparison of Activity with Designed, Cationicity-Enhanced Analogues and Diastereomers.Molecules. 2016 Dec 3;21(12):1667. doi: 10.3390/molecules21121667. Molecules. 2016. PMID: 27918477 Free PMC article.
-
Dermaseptins as models for the elucidation of membrane-acting helical amphipathic antimicrobial peptides.Curr Pharm Biotechnol. 2011 Aug;12(8):1184-93. doi: 10.2174/138920111796117319. Curr Pharm Biotechnol. 2011. PMID: 21470155 Review.
-
Structural diversity and species distribution of host-defense peptides in frog skin secretions.Cell Mol Life Sci. 2011 Jul;68(13):2303-15. doi: 10.1007/s00018-011-0720-8. Epub 2011 May 11. Cell Mol Life Sci. 2011. PMID: 21560068 Free PMC article. Review.
Cited by
-
Phylloseptin-1 is Leishmanicidal for Amastigotes of Leishmaniaamazonensis Inside Infected Macrophages.Int J Environ Res Public Health. 2020 Jul 6;17(13):4856. doi: 10.3390/ijerph17134856. Int J Environ Res Public Health. 2020. PMID: 32640562 Free PMC article.
-
Neglected Venomous Animals and Toxins: Underrated Biotechnological Tools in Drug Development.Toxins (Basel). 2021 Nov 29;13(12):851. doi: 10.3390/toxins13120851. Toxins (Basel). 2021. PMID: 34941689 Free PMC article. Review.
-
Phylloseptin-PBa--A Novel Broad-Spectrum Antimicrobial Peptide from the Skin Secretion of the Peruvian Purple-Sided Leaf Frog (Phyllomedusa Baltea) Which Exhibits Cancer Cell Cytotoxicity.Toxins (Basel). 2015 Dec 1;7(12):5182-93. doi: 10.3390/toxins7124878. Toxins (Basel). 2015. PMID: 26633506 Free PMC article.
-
Sarkosyl-Induced Helical Structure of an Antimicrobial Peptide GW-Q6 Plays an Essential Role in the Binding of Surface Receptor OprI in Pseudomonas aeruginosa.PLoS One. 2016 Oct 11;11(10):e0164597. doi: 10.1371/journal.pone.0164597. eCollection 2016. PLoS One. 2016. PMID: 27727309 Free PMC article.
-
Identification of novel antimicrobial peptide from Asian sea bass (Lates calcarifer) by in silico and activity characterization.PLoS One. 2018 Oct 26;13(10):e0206578. doi: 10.1371/journal.pone.0206578. eCollection 2018. PLoS One. 2018. PMID: 30365554 Free PMC article.
References
-
- Nicolas P, El Amri C (2009) The dermaseptin superfamily: a gene-based combinatorial library of antimicrobial peptides. Biochim Biophys Acta 1788: 1537–1550. - PubMed
-
- Nicolas P, Ladram A (2013) Dermaseptins. In: Kastin AJ, editor, Handbook of Biologically Active Peptides, Second edition. Elsevier. 350–363.
-
- Vanhoye D, Bruston F, Nicolas P, Amiche M (2003) Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur J Biochem 270: 2068–2081. - PubMed
-
- Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462: 55–70. - PubMed
-
- Lequin O, Bruston F, Convert O, Chassaing G, Nicolas P (2003) Helical structure of dermaseptin B2 in a membrane-mimetic environment. Biochemistry 42: 10311–10323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

