Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix
- PMID: 23967136
- PMCID: PMC3744544
- DOI: 10.1371/journal.pone.0070916
Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix
Abstract
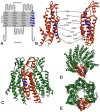
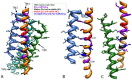
Human Connexin26 gene mutations cause hearing loss. These hereditary mutations are the leading cause of childhood deafness worldwide. Mutations in gap junction proteins (connexins) can impair intercellular communication by eliminating protein synthesis, mis-trafficking, or inducing channels that fail to dock or have aberrant function. We previously identified a new class of mutants that form non-functional gap junction channels and hemichannels (connexons) by disrupting packing and inter-helix interactions. Here we analyzed fourteen point mutations in the fourth transmembrane helix of connexin26 (Cx26) that cause non-syndromic hearing loss. Eight mutations caused mis-trafficking (K188R, F191L, V198M, S199F, G200R, I203K, L205P, T208P). Of the remaining six that formed gap junctions in mammalian cells, M195T and A197S formed stable hemichannels after isolation with a baculovirus/Sf9 protein purification system, while C202F, I203T, L205V and N206S formed hemichannels with varying degrees of instability. The function of all six gap junction-forming mutants was further assessed through measurement of dye coupling in mammalian cells and junctional conductance in paired Xenopus oocytes. Dye coupling between cell pairs was reduced by varying degrees for all six mutants. In homotypic oocyte pairings, only A197S induced measurable conductance. In heterotypic pairings with wild-type Cx26, five of the six mutants formed functional gap junction channels, albeit with reduced efficiency. None of the mutants displayed significant alterations in sensitivity to transjunctional voltage or induced conductive hemichannels in single oocytes. Intra-hemichannel interactions between mutant and wild-type proteins were assessed in rescue experiments using baculovirus expression in Sf9 insect cells. Of the four unstable mutations (C202F, I203T, L205V, N206S) only C202F and N206S formed stable hemichannels when co-expressed with wild-type Cx26. Stable M195T hemichannels displayed an increased tendency to aggregate. Thus, mutations in TM4 cause a range of phenotypes of dysfunctional gap junction channels that are discussed within the context of the X-ray crystallographic structure.
Conflict of interest statement
Figures








References
-
- Sohl G, Willecke K (2004) Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232. - PubMed
-
- Harris A, Locke D (2009) Connexins : a guide. New York, N.Y.: Springer. xvi, 573 p.
-
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400. - PubMed
-
- Evans WH, Martin PE (2002) Gap junctions: structure and function (Review). Mol Membr Biol 19: 121–136. - PubMed
-
- Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, et al. (2009) Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458: 597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

