SNPs altering ammonium transport activity of human Rhesus factors characterized by a yeast-based functional assay
- PMID: 23967154
- PMCID: PMC3742762
- DOI: 10.1371/journal.pone.0071092
SNPs altering ammonium transport activity of human Rhesus factors characterized by a yeast-based functional assay
Abstract
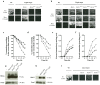
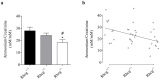
Proteins of the conserved Mep-Amt-Rh family, including mammalian Rhesus factors, mediate transmembrane ammonium transport. Ammonium is an important nitrogen source for the biosynthesis of amino acids but is also a metabolic waste product. Its disposal in urine plays a critical role in the regulation of the acid/base homeostasis, especially with an acid diet, a trait of Western countries. Ammonium accumulation above a certain concentration is however pathologic, the cytotoxicity causing fatal cerebral paralysis in acute cases. Alteration in ammonium transport via human Rh proteins could have clinical outcomes. We used a yeast-based expression assay to characterize human Rh variants resulting from non synonymous single nucleotide polymorphisms (nsSNPs) with known or unknown clinical phenotypes and assessed their ammonium transport efficiency, protein level, localization and potential trans-dominant impact. The HsRhAG variants (I61R, F65S) associated to overhydrated hereditary stomatocytosis (OHSt), a disease affecting erythrocytes, proved affected in intrinsic bidirectional ammonium transport. Moreover, this study reveals that the R202C variant of HsRhCG, the orthologue of mouse MmRhcg required for optimal urinary ammonium excretion and blood pH control, shows an impaired inherent ammonium transport activity. Urinary ammonium excretion was RHcg gene-dose dependent in mouse, highlighting MmRhcg as a limiting factor. HsRhCG(R202C) may confer susceptibility to disorders leading to metabolic acidosis for instance. Finally, the analogous R211C mutation in the yeast ScMep2 homologue also impaired intrinsic activity consistent with a conserved functional role of the preserved arginine residue. The yeast expression assay used here constitutes an inexpensive, fast and easy tool to screen nsSNPs reported by high throughput sequencing or individual cases for functional alterations in Rh factors revealing potential causal variants.
Conflict of interest statement
Figures




References
-
- Auron A, Brophy PD (2012) Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatric nephrology 27: 207–222. - PubMed
-
- Adeva MM, Souto G (2011) Diet-induced metabolic acidosis. Clinical nutrition 30: 416–421. - PubMed
-
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, et al. (2005) Origins and evolution of the Western diet: health implications for the 21st century. The American journal of clinical nutrition 81: 341–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

