Beneficial impact of CCL2 and CCL12 neutralization on experimental malignant pleural effusion
- PMID: 23967166
- PMCID: PMC3743892
- DOI: 10.1371/journal.pone.0071207
Beneficial impact of CCL2 and CCL12 neutralization on experimental malignant pleural effusion
Abstract
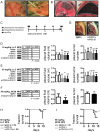
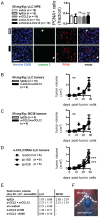
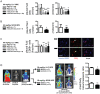
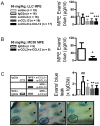
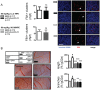
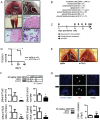
Using genetic interventions, we previously determined that C-C motif chemokine ligand 2 (CCL2) promotes malignant pleural effusion (MPE) formation in mice. Here we conducted preclinical studies aimed at assessing the specific therapeutic potential of antibody-mediated CCL2 blockade against MPE. For this, murine MPEs or skin tumors were generated in C57BL/6 mice by intrapleural or subcutaneous delivery of lung (LLC) or colon (MC38) adenocarcinoma cells. Human lung adenocarcinoma cells (A549) were used to induce MPEs in severe combined immunodeficient mice. Intraperitoneal antibodies neutralizing mouse CCL2 and/or CCL12, a murine CCL2 ortholog, were administered at 10 or 50 mg/kg every three days. We found that high doses of CCL2/12 neutralizing antibody treatment (50 mg/kg) were required to limit MPE formation by LLC cells. CCL2 and CCL12 blockade were equally potent inhibitors of MPE development by LLC cells. Combined CCL2 and CCL12 neutralization was also effective against MC38-induced MPE and prolonged the survival of mice in both syngeneic models. Mouse-specific CCL2-blockade limited A549-caused xenogeneic MPE, indicating that host-derived CCL2 also contributes to MPE precipitation in mice. The impact of CCL2/12 antagonism was associated with inhibition of immune and vascular MPE-related phenomena, such as inflammation, new blood vessel assembly and plasma extravasation into the pleural space. We conclude that CCL2 and CCL12 blockade are effective against experimental MPE induced by murine and human adenocarcinoma in mice. These results suggest that CCL2-targeted therapies may hold promise for future use against human MPE.
Conflict of interest statement
Figures
References
-
- Maskell NA (2012) Treatment options for malignant pleural effusions: patient preference does matter. JAMA 307: 2432–2433. - PubMed
-
- Wu SG, Yu CJ, Tsai MF, Liao WY, Yang CH, et al.. (2012) Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J, In press. - PubMed
-
- Burgers JA, Kunst PW, Koolen MG, Willems LN, Burgers JS, et al. (2008) Pleural drainage and pleurodesis: implementation of guidelines in four hospitals. Eur Respir J 32: 1321–1327. - PubMed
-
- Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, et al. (2012) Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 307: 2383–2389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

