Fibroblast growth factor-4 enhances proliferation of mouse embryonic stem cells via activation of c-Jun signaling
- PMID: 23967228
- PMCID: PMC3742512
- DOI: 10.1371/journal.pone.0071641
Fibroblast growth factor-4 enhances proliferation of mouse embryonic stem cells via activation of c-Jun signaling
Abstract
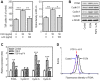
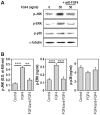
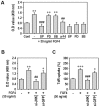
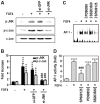
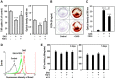
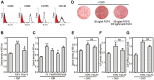
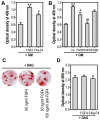
Fibroblast growth factor-4 (FGF4) is expressed in embryonic stages and in adult tissues, where it plays critical roles in modulating multiple cellular functions. However, the exact roles of FGF4 on proliferation and differentiation of embryonic stem cells (ESCs) are not completely understood. Exogenous addition of FGF4 stimulated proliferation of mouse ESCs (mESCs), as proven by the increases in DNA synthesis and cell cycle regulatory protein induction. These increases were almost completely inhibited by pre-treating cells with anti-FGF4 antibody. FGF4 also activated c-Jun N-terminal kinase (JNK) and extracellular-signal regulated kinase (ERK) signaling, but not p38 kinase. Blockage of JNK signaling by SP600125 or by transfection with its specific siRNA significantly inhibited FGF4-stimulated cell proliferation through the suppression of c-Jun induction and activator protein-1 (AP-1) activity. However, ERK or p38 kinase inhibitor did not affect FGF4-stimulated proliferation in mESCs. FGF4 suppressed osteogenic differentiation of mESCs by inhibiting expression of transcription factors involved in bone formation. Further, exogenous FGF4 addition stimulated proliferation of human periodontal ligament stem cells (hPDLSCs) and bone marrow mesenchymal stem cells (BMMSCs) via activation of ERK signaling. FGF4 also augmented mineralization of hPDLSCs, but not of BMMSCs. Collectively, it is suggested that FGF4 triggers proliferation of stem cells by activating MAPK-mediated signaling, while it affects differently osteogenic differentiation according to the origins of stem cells.
Conflict of interest statement
Figures


References
-
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair and Regeneration 16: 585–601. - PubMed
-
- D’Andrea LD, Del Gatto A, De Rosa L, Romanelli A, Pedone C (2009) Peptides targeting angiogenesis related growth factor receptors. Current Pharmaceutical Design 15: 2414–2429. - PubMed
-
- Quarto N, Wan DC, Longaker MT (2008) Molecular mechanisms of FGF-2 inhibitory activity in the osteogenic context of mouse adipose-derived stem cells (mASCs). Bone 42: 1040–1052. - PubMed
-
- Ahn HJ, Lee WJ, Kwack K, Kwon YD (2009) FGF2 stimulates the proliferation of human mesenchymal stem cells through the transient activation of JNK signaling. FEBS Letters 583: 2922–2926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

