Vascular permeability and drug delivery in cancers
- PMID: 23967403
- PMCID: PMC3744053
- DOI: 10.3389/fonc.2013.00211
Vascular permeability and drug delivery in cancers
Abstract
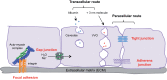
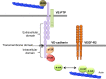
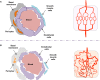
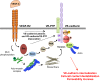
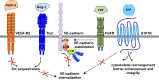
The endothelial barrier strictly maintains vascular and tissue homeostasis, and therefore modulates many physiological processes such as angiogenesis, immune responses, and dynamic exchanges throughout organs. Consequently, alteration of this finely tuned function may have devastating consequences for the organism. This is particularly obvious in cancers, where a disorganized and leaky blood vessel network irrigates solid tumors. In this context, vascular permeability drives tumor-induced angiogenesis, blood flow disturbances, inflammatory cell infiltration, and tumor cell extravasation. This can directly restrain the efficacy of conventional therapies by limiting intravenous drug delivery. Indeed, for more effective anti-angiogenic therapies, it is now accepted that not only should excessive angiogenesis be alleviated, but also that the tumor vasculature needs to be normalized. Recovery of normal state vasculature requires diminishing hyperpermeability, increasing pericyte coverage, and restoring the basement membrane, to subsequently reduce hypoxia, and interstitial fluid pressure. In this review, we will introduce how vascular permeability accompanies tumor progression and, as a collateral damage, impacts on efficient drug delivery. The molecular mechanisms involved in tumor-driven vascular permeability will next be detailed, with a particular focus on the main factors produced by tumor cells, especially the emblematic vascular endothelial growth factor. Finally, new perspectives in cancer therapy will be presented, centered on the use of anti-permeability factors and normalization agents.
Keywords: VE-cadherin; VEGF; endothelial barrier; permeability; tumor angiogenesis.
Figures
References
-
- Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, et al. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem (2002) 277:40091–8 10.1074/jbc.M205948200 - DOI - PubMed
-
- Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol (2003) 285:L1179–83 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

