3,4-Dichlorophenoxyacetate interleaved into anionic clay for controlled release formulation of a new environmentally friendly agrochemical
- PMID: 23968197
- PMCID: PMC3765951
- DOI: 10.1186/1556-276X-8-362
3,4-Dichlorophenoxyacetate interleaved into anionic clay for controlled release formulation of a new environmentally friendly agrochemical
Abstract
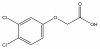
A new layered organic-inorganic nanohybrid material, zinc-aluminum-3,4-dicholorophenoxyacetate (N3,4-D) in which an agrochemical, 3,4-dichlorophenoxyacetic acid (3,4-D), is intercalated into zinc-aluminum-layered double hydroxide (ZAL), was synthesized by coprecipitation method. A well-ordered nanomaterial was formed with a percentage loading of 53.5% (w/w). Due to the inclusion of 3,4-D, basal spacing expanded from 8.9 Å in ZAL to 18.7 Å in N3,4-D. The Fourier transform infrared study shows that the absorption bands of the resulting nanohybrid composed of both the 3,4-D and ZAL further confirmed the intercalation episode. Thermal analysis shows that ZAL host enhances the thermal stability of 3,4-D. Controlled-release experiment shows that the release of 3,4-D in the aqueous media is in the order of phosphate > carbonate > sulfate > chloride. These studies demonstrate the successful intercalation of the 3,4-D and its controlled release property in various aqueous media.
Figures







References
-
- Gish TJ, Scoppet MJ, Helling CS, Schirmohammadi A, Schenecher MM, Wing RE. Transport comparison of technical grade and starch-encapsulated atrazine. Trans ASAE. 2011;8:1738–17444.
-
- Srivastava B, Patanjali PK, Basu V, Jhelum DD. Adsorbents for pesticide uptake from contaminated water: a review. J Sci Ind Res. 2009;8:839–850.
-
- Derylo-Marczewska AM, ABlachnio W, Marczewski B, Tarasiuk. Adsorption of selected herbicides from aqueous solutions on activated carbon. J Therm Anal Calorim. 2010;8:785–794. doi: 10.1007/s10973-010-0840-7. - DOI
-
- Modabber Ahmed K, Choong-Lyeal C, Dong-Hoon L, Man P, Bu-Kug L, Jong-Yoon L, Jyung-Choi. Synthesis and properties of mecoprop-intercalated layered double hydroxide. J Phys Chem Solids. 2007;8:1591–1597. doi: 10.1016/j.jpcs.2007.03.045. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

