2,2-Diphenyl-1-picrylhydrazyl as a screening tool for recombinant monoterpene biosynthesis
- PMID: 23968454
- PMCID: PMC3847554
- DOI: 10.1186/1475-2859-12-76
2,2-Diphenyl-1-picrylhydrazyl as a screening tool for recombinant monoterpene biosynthesis
Abstract
Background: Monoterpenes are a class of natural C10 compounds with a range of potential applications including use as fuel additives, fragrances, and chemical feedstocks. Biosynthesis of monoterpenes in heterologous systems is yet to reach commercially-viable levels, and therefore is the subject of strain engineering and fermentation optimization studies. Detection of monoterpenes typically relies on gas chromatography/mass spectrometry; this represents a significant analytical bottleneck which limits the potential to analyse combinatorial sets of conditions. To address this, we developed a high-throughput method for pre-screening monoterpene biosynthesis.
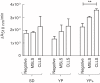
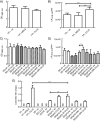
Results: An optimised DPPH assay was developed for detecting monoterpenes from two-phase microbial cultures using dodecane as the extraction solvent. The assay was useful for reproducible qualitative ranking of monoterpene concentrations, and detected standard preparations of myrcene and γ-terpinene dissolved in dodecane at concentrations as low as 10 and 15 μM, respectively, and limonene as low as 200 μM. The assay could not be used quantitatively due to technical difficulties in capturing the initial reaction rate in a multi-well plate and the presence of minor DPPH-reactive contaminants. Initially, limonene biosynthesis in Saccharomyces cerevisiae was tested using two different limonene synthase enzymes and three medium compositions. The assay indicated that limonene biosynthesis was enhanced in a supplemented YP medium and that the Citrus limon limonene synthase (CLLS) was more effective than the Mentha spicata limonene synthase (MSLS). GC-MS analysis revealed that the DPPH assay had correctly identified the best limonene synthase (CLLS) and culture medium (supplemented YP medium). Because only traces of limonene were detected in SD medium, we subsequently identified medium components that improved limonene production and developed a defined medium based on these findings. The best limonene titres obtained were 1.48 ± 0.22 mg limonene per L in supplemented YP medium and 0.9 ± 0.15 mg limonene per L in a pH-adjusted supplemented SD medium.
Conclusions: The DPPH assay is useful for detecting biosynthesis of limonene. Although the assay cannot be used quantitatively, it proved successful in ranking limonene production conditions qualitatively and thus is suitable as a first-tier screen. The DPPH assay will likely be applicable in detecting biosynthesis of several other monoterpenes and for screening libraries of monoterpene-producing strains.
Figures


References
-
- Keasling JD. In: Handbook of hydrocarbon and lipid microbiology. Timmis KN, editor. Berlin Heidelberg: Springer; 2010. Microbial production of isoprenoids; pp. 2951–2966.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

