3,3'-diindolylmethane rapidly and selectively inhibits hepatocyte growth factor/c-Met signaling in breast cancer cells
- PMID: 23968581
- PMCID: PMC3808088
- DOI: 10.1016/j.jnutbio.2013.05.004
3,3'-diindolylmethane rapidly and selectively inhibits hepatocyte growth factor/c-Met signaling in breast cancer cells
Abstract
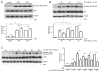
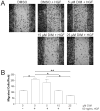
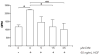
3,3'-Diindolylmethane (DIM), an indole derivative from vegetables of the Brassica genus, has antiproliferative activity in breast cancer cells. Part of this activity is thought to be due to DIM inhibition of Akt signaling, but an upstream mechanism of DIM-induced Akt inhibition has not been described. The goals of this study were to investigate the kinetics of inhibition of Akt by physiologically relevant concentrations of DIM and to identify an upstream factor that mediates this effect. Here we report that DIM (5-25 μM) inhibited Akt activation from 30 min to 24h in tumorigenic MDA-MB-231 cells but did not inhibit Akt activation in non-tumorigenic preneoplastic MCF10AT cells. DIM inhibited hepatocyte growth factor (HGF)-induced Akt activation by up to 46%, cell migration by 66% and cell proliferation by up to 54%, but did not inhibit induction of Akt by epidermal growth factor or insulin-like growth factor-1. DIM decreased phosphorylation of the HGF receptor, c-Met, at tyrosines 1234 and 1235, indicating decreased activation of the receptor. This decrease was reversed by pretreatment with inhibitors of p38 or calcineurin. Our results demonstrate the important role of HGF and c-Met in DIM's anti-proliferative effect on breast cancer cells and suggest that DIM could have preventive or clinical value as an inhibitor of c-Met signaling.
Keywords: 3,3′-diindolylmethane; Akt; Breast cancer; c-Met.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: None
Figures


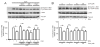
References
-
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. Oxford; New York: Oxford University Press; 1981. - PubMed
-
- WCRF/AICR. Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. Washington, DC: 1997.
-
- Negri E, Lavecchia C, Franceschi S, Davanzo B, Parazzini F. Vegetable and Fruit Consumption and Cancer Risk. International Journal of Cancer. 1991;48:350–4. - PubMed
-
- Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. JAMA. 2001;285:2975–7. - PubMed
-
- Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

