Influence of electron dose rate on electron counting images recorded with the K2 camera
- PMID: 23968652
- PMCID: PMC3854003
- DOI: 10.1016/j.jsb.2013.08.005
Influence of electron dose rate on electron counting images recorded with the K2 camera
Abstract
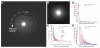
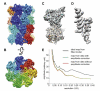
A recent technological breakthrough in electron cryomicroscopy (cryoEM) is the development of direct electron detection cameras for data acquisition. By bypassing the traditional phosphor scintillator and fiber optic coupling, these cameras have greatly enhanced sensitivity and detective quantum efficiency (DQE). Of the three currently available commercial cameras, the Gatan K2 Summit was designed specifically for counting individual electron events. Counting further enhances the DQE, allows for practical doubling of detector resolution and eliminates noise arising from the variable deposition of energy by each primary electron. While counting has many advantages, undercounting of electrons happens when more than one electron strikes the same area of the detector within the analog readout period (coincidence loss), which influences image quality. In this work, we characterized the K2 Summit in electron counting mode, and studied the relationship of dose rate and coincidence loss and its influence on the quality of counted images. We found that coincidence loss reduces low frequency amplitudes but has no significant influence on the signal-to-noise ratio of the recorded image. It also has little influence on high frequency signals. Images of frozen hydrated archaeal 20S proteasome (~700 kDa, D7 symmetry) recorded at the optimal dose rate retained both high-resolution signal and low-resolution contrast and enabled calculating a 3.6 Å three-dimensional reconstruction from only 10,000 particles.
Keywords: Direct detection camera; Electron cryomicroscopy; Single particle.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Avila-Sakar A, Li X, Zheng SQ, Cheng Y. Recording high-resoution images of two-dimensional crystals of membrane proteins. In: Schmid-Krey I, Cheng Y, editors. Electron Crystallography of Soluble and Membrane Proteins, Methods and Protocols. Humana Press; 2013. pp. 129–152. - PubMed
-
- Booth CR, Jakana J, Chiu W. Assessing the capabilities of a 4k × 4k CCD camera for electron cryo-microscopy at 300 kV. Journal of Structural ~Biology. 2006;156:556–563. - PubMed
-
- Carragher B, Kisseberth N, Kriegman D, Milligan RA, Potter CS, Pulokas J, Reilein A. Leginon: an automated system for acquisition of images from vitreous ice specimens. Journal of Structural Biology. 2000;132:33–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

