A forward genetic screen in mice identifies mutants with abnormal cortical patterning
- PMID: 23968836
- PMCID: PMC4259276
- DOI: 10.1093/cercor/bht209
A forward genetic screen in mice identifies mutants with abnormal cortical patterning
Abstract
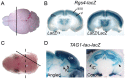
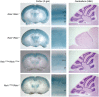
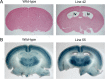
Formation of a 6-layered cortical plate and axon tract patterning are key features of cerebral cortex development. Abnormalities of these processes may be the underlying cause for a range of functional disabilities seen in human neurodevelopmental disorders. To identify mouse mutants with defects in cortical lamination or corticofugal axon guidance, N-ethyl-N-nitrosourea (ENU) mutagenesis was performed using mice expressing LacZ reporter genes in layers II/III and V of the cortex (Rgs4-lacZ) or in corticofugal axons (TAG1-tau-lacZ). Four lines with abnormal cortical lamination have been identified. One of these was a splice site mutation in reelin (Reln) that results in a premature stop codon and the truncation of the C-terminal region (CTR) domain of reelin. Interestingly, this novel allele of Reln did not display cerebellar malformation or ataxia, and this is the first report of a Reln mutant without a cerebellar defect. Four lines with abnormal cortical axon development were also identified, one of which was found by whole-genome resequencing to carry a mutation in Lrp2. These findings demonstrated that the application of ENU mutagenesis to mice carrying transgenic reporters marking cortical anatomy is a sensitive and specific method to identify mutations that disrupt patterning of the developing brain.
Keywords: ENU mutagenesis; cerebral cortex; cortical lamination; corticofugal axon; reelin.
© The Author 2013. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures







References
-
- Anderson KV. Finding the genes that direct mammalian development : ENU mutagenesis in the mouse. Trends Genet. 2000;16:99–102. - PubMed
-
- Andersen TE, Finsen B, Goffinet AM, Issinger OG, Boldyreff B. A reeler mutant mouse with a new, spontaneous mutation in the reelin gene. Brain Res Mol Brain Res. 2002;105:153–156. - PubMed
-
- Beier DR, Herron BJ. Genetic mapping and ENU mutagenesis. Genetica. 2004;122:65–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

