Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: redundant roles for PiT-1 and PiT-2
- PMID: 23968976
- PMCID: PMC4009978
- DOI: 10.1161/ATVBAHA.113.302249
Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: redundant roles for PiT-1 and PiT-2
Abstract
Objective: Elevated serum phosphate has emerged as a major risk factor for vascular calcification. The sodium-dependent phosphate cotransporter, PiT-1, was previously shown to be required for phosphate-induced osteogenic differentiation and calcification of cultured human vascular smooth muscle cells (VSMCs), but its importance in vascular calcification in vivo and the potential role of its homologue, PiT-2, have not been determined. We investigated the in vivo requirement for PiT-1 in vascular calcification using a mouse model of chronic kidney disease and the potential compensatory role of PiT-2 using in vitro knockdown and overexpression strategies.
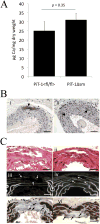
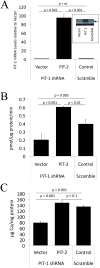
Approach and results: Mice with targeted deletion of PiT-1 in VSMCs were generated (PiT-1(Δsm)). PiT-1 mRNA levels were undetectable, whereas PiT-2 mRNA levels were increased 2-fold in the vascular aortic media of PiT-1(Δsm) compared with PiT-1(flox/flox) control. When arterial medial calcification was induced in PiT-1(Δsm) and PiT-1(flox/flox) by chronic kidney disease followed by dietary phosphate loading, the degree of aortic calcification was not different between genotypes, suggesting compensation by PiT-2. Consistent with this possibility, VSMCs isolated from PiT-1(Δsm) mice had no PiT-1 mRNA expression, increased PiT-2 mRNA levels, and no difference in sodium-dependent phosphate uptake or phosphate-induced matrix calcification compared with PiT-1(flox/flox) VSMCs. Knockdown of PiT-2 decreased phosphate uptake and phosphate-induced calcification of PiT-1(Δsm) VSMCs. Furthermore, overexpression of PiT-2 restored these parameters in human PiT-1-deficient VSMCs.
Conclusions: PiT-2 can mediate phosphate uptake and calcification of VSMCs in the absence of PiT-1. Mechanistically, PiT-1 and PiT-2 seem to serve redundant roles in phosphate-induced calcification of VSMCs.
Keywords: PiT-1; PiT-2; phosphate; vascular calcification; vascular smooth muscle cell.
Figures




References
-
- U.S. Renal data system (usrds) 2011 annual data report. Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: http://www.usrds.org/adr.htm.
-
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. - PubMed
-
- Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. - PubMed
-
- Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

