From tissue mechanics to transcription factors
- PMID: 23969122
- PMCID: PMC4545622
- DOI: 10.1016/j.diff.2013.07.004
From tissue mechanics to transcription factors
Abstract
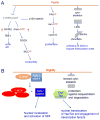
Changes in tissue stiffness are frequently associated with diseases such as cancer, fibrosis, and atherosclerosis. Several recent studies suggest that, in addition to resulting from pathology, mechanical changes may play a role akin to soluble factors in causing the progression of disease, and similar mechanical control might be essential for normal tissue development and homeostasis. Many cell types alter their structure and function in response to exogenous forces or as a function of the mechanical properties of the materials to which they adhere. This review summarizes recent progress in identifying intracellular signaling pathways, and especially transcriptional programs, that are differentially activated when cells adhere to materials with different mechanical properties or when they are subject to tension arising from external forces. Several cytoplasmic or cytoskeletal signaling pathways involving small GTPases, focal adhesion kinase and transforming growth factor beta as well as the transcriptional regulators MRTF-A, NFκB, and Yap/Taz have emerged as important mediators of mechanical signaling.
Keywords: Mechanical stress; Mechanosensing; Mechanotransduction; Substrate stiffness.
© 2013 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integrative Biology. 2012;4:410–421. - PubMed
-
- Blank RS, McQuinn TC, Yin KC, Thompson MM, Takeyasu K, Schwartz RJ, Owens GK. Elements of the smooth muscle alpha-actin promoter required in cis for transcriptional activation in smooth muscle. Evidence for cell type-specific regulation. Journal of Biological Chemistry. 1992;267:984–989. - PubMed
-
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. American Journal of Respiratory and Critical Care Medicine. 2012;186:866–876. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

