Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases
- PMID: 23969216
- PMCID: PMC4673667
- DOI: 10.1016/S1473-3099(13)70190-7
Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases
Abstract
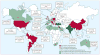
Klebsiella pneumoniae carbapenemases (KPCs) were originally identified in the USA in 1996. Since then, these versatile β-lactamases have spread internationally among Gram-negative bacteria, especially K pneumoniae, although their precise epidemiology is diverse across countries and regions. The mortality described among patients infected with organisms positive for KPC is high, perhaps as a result of the limited antibiotic options remaining (often colistin, tigecycline, or aminoglycosides). Triple drug combinations using colistin, tigecycline, and imipenem have recently been associated with improved survival among patients with bacteraemia. In this Review, we summarise the epidemiology of KPCs across continents, and discuss issues around detection, present antibiotic options and those in development, treatment outcome and mortality, and infection control. In view of the limitations of present treatments and the paucity of new drugs in the pipeline, infection control must be our primary defence for now.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
LSM-P has received speaking fees from Sage and Steris. LP and PN have received research funds from Merck. MG’s work was partially financed by the grant Narodowy Program Ochrony Antybiotykow (NPOA) from the Polish Ministry of Health, and the grant SPUB MIKROBANK from the Polish Ministry of Science and Higher Education. DML has shareholdings in Dechra, Eco Animal Health, Merck, and Pfizer; has accepted grants, speaking invitations, and conference invitations from Pfizer, Novartis, AstraZeneca, and Astellas; and has advisory or consultancy relationships with Achaogen, AstraZeneca, Basilea, Bayer, Cubist, Curetis, Discuva, GlaxoSmithKline, Kalidex, McKinsey, Meiji, Pfizer, Roche, Tetraphase, Theravance, and Wockhardt. JP has received research funds from Merck and AstraZeneca. MVV has received research grants from Merck Sharp & Dohme, Pfizer SA, Janssen-Cilag SA, Novartis, Merck Colombia, AstraZeneca Colombia SA, bioMerieux Colombia SAS, and Colciencias. JPQ is an employee and potential shareholder in AstraZeneca. All other authors declare that they have no conflicts of interest.
Figures
Comment in
-
Clinical epidemiology of Klebsiella pneumoniae carbapenemases.Lancet Infect Dis. 2014 Apr;14(4):271-2. doi: 10.1016/S1473-3099(14)70705-4. Lancet Infect Dis. 2014. PMID: 24670625 No abstract available.
References
-
- Bradford PA, Bratu S, Urban C, et al. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin Infect Dis. 2004;39:55–60. - PubMed
-
- Chiang T, Mariano N, Urban C, et al. Identification of carbapenem-resistant Klebsiella pneumoniae harboring KPC enzymes in New Jersey. Microb Drug Resist. 2007;13:235–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources