Survival in women with grade 1 serous ovarian carcinoma
- PMID: 23969788
- PMCID: PMC4870595
- DOI: 10.1097/AOG.0b013e31829ce7ec
Survival in women with grade 1 serous ovarian carcinoma
Abstract
Objective: To examine clinicopathologic variables associated with survival among women with low-grade (grade 1) serous ovarian carcinoma enrolled in a phase III study.
Methods: This was an ancillary data analysis of Gynecologic Oncology Group protocol 182, a phase III study of women with stage III-IV epithelial ovarian carcinoma treated with carboplatin and paclitaxel compared with triplet or sequential doublet regimens. Women with grade 1 serous carcinoma (a surrogate for low-grade serous disease) were included in the analysis.
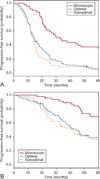
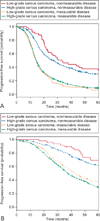
Results: Among the 3,686 enrolled participants, 189 had grade 1 disease. The median age was 56.5 years and 87.3% had stage III disease. The median follow-up time was 47.1 months. Stratification according to residual disease after primary surgery was microscopic residual in 24.9%, 0.1-1.0 cm of residual in 51.3%, and more than 1.0 cm of residual in 23.8%. On multivariate analysis, only residual disease status (P=.006) was significantly associated with survival. Patients with microscopic residual had a significantly longer median progression-free (33.2 months) and overall survival (96.9 months) compared with those with residual 0.1-1.0 cm (14.7 months and 44.5 months, respectively) and more than 1.0 cm of residual disease (14.1 months and 42.0 months, respectively; progression-free and overall survival, P<.001). After adjustment for other variables, patients with low-grade serous carcinoma with measurable residual disease had a similar adjusted hazard ratio for death (2.12; P=.002) as their high-grade serous carcinoma counterparts with measurable disease (2.31; P<.001).
Conclusions: Surgical cytoreduction to microscopic residual was associated with improved progression-free and overall survival in women with advanced-stage low-grade serous ovarian carcinoma.
Clinical trial registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00011986.
Level of evidence: II.
Conflict of interest statement
The authors did not report any potential conflicts of interest.
Figures
References
-
- Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. - PubMed
-
- Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. - PubMed
-
- Malpica A, Deavers MT, Tornos C, Kurman RJ, Soslow R, Seidman JD, et al. Interobserver and intraobserver variability of a two-tier system for grading ovarian serous carcinoma. Am J Surg Pathol. 2007;31:1168–1174. - PubMed
-
- Gershenson DM, Sun CC, Lu KH, Coleman RL, Ak Sood, Malpica A, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;2:361–368. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

