Withaferin A: a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone
- PMID: 23969857
- PMCID: PMC3763455
- DOI: 10.1038/cddis.2013.294
Withaferin A: a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone
Abstract
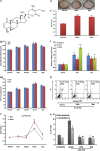
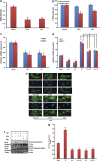
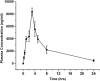
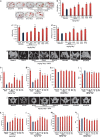
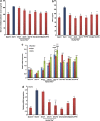
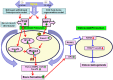
Withania somnifera or Ashwagandha is a medicinal herb of Ayurveda. Though the extract and purified molecules, withanolides, from this plant have been shown to have different pharmacological activities, their effect on bone formation has not been studied. Here, we show that one of the withanolide, withaferin A (WFA) acts as a proteasomal inhibitor (PI) and binds to specific catalytic β subunit of the 20S proteasome. It exerts positive effect on osteoblast by increasing osteoblast proliferation and differentiation. WFA increased expression of osteoblast-specific transcription factor and mineralizing genes, promoted osteoblast survival and suppressed inflammatory cytokines. In osteoclast, WFA treatment decreased osteoclast number directly by decreasing expression of tartarate-resistant acid phosphatase and receptor activator of nuclear factor kappa-B (RANK) and indirectly by decreasing osteoprotegrin/RANK ligand ratio. Our data show that in vitro treatment of WFA to calvarial osteoblast cells decreased expression of E3 ubiquitin ligase, Smad ubiquitin regulatory factor 2 (Smurf2), preventing degradation of Runt-related transcription factor 2 (RunX2) and relevant Smad proteins, which are phosphorylated by bone morphogenetic protein 2. Increased Smurf2 expression due to exogenous treatment of tumor necrosis factor α (TNFα) to primary osteoblast cells was decreased by WFA treatment. This was corroborated by using small interfering RNA against Smurf2. Further, WFA also blocked nuclear factor kappa-B (NF-kB) signaling as assessed by tumor necrosis factor stimulated nuclear translocation of p65-subunit of NF-kB. Overall data show that in vitro proteasome inhibition by WFA simultaneously promoted osteoblastogenesis by stabilizing RunX2 and suppressed osteoclast differentiation, by inhibiting osteoclastogenesis. Oral administration of WFA to osteopenic ovariectomized mice increased osteoprogenitor cells in the bone marrow and increased expression of osteogenic genes. WFA supplementation improved trabecular micro-architecture of the long bones, increased biomechanical strength parameters of the vertebra and femur, decreased bone turnover markers (osteocalcin and TNFα) and expression of skeletal osteoclastogenic genes. It also increased new bone formation and expression of osteogenic genes in the femur bone as compared with vehicle groups (Sham) and ovariectomy (OVx), Bortezomib (known PI), injectible parathyroid hormone and alendronate (FDA approved drugs). WFA promoted the process of cortical bone regeneration at drill-holes site in the femur mid-diaphysis region and cortical gap was bridged with woven bone within 11 days of both estrogen sufficient and deficient (ovariectomized, Ovx) mice. Together our data suggest that WFA stimulates bone formation by abrogating proteasomal machinery and provides knowledge base for its clinical evaluation as a bone anabolic agent.
Figures


Similar articles
-
Preventive effects of withaferin A isolated from the leaves of an Indian medicinal plant Withania somnifera (L.): comparisons with 17-β-estradiol and alendronate.Nutrition. 2015 Jan;31(1):205-13. doi: 10.1016/j.nut.2014.05.010. Epub 2014 Jun 10. Nutrition. 2015. PMID: 25466667
-
A novel quercetin analogue from a medicinal plant promotes peak bone mass achievement and bone healing after injury and exerts an anabolic effect on osteoporotic bone: the role of aryl hydrocarbon receptor as a mediator of osteogenic action.J Bone Miner Res. 2011 Sep;26(9):2096-111. doi: 10.1002/jbmr.434. J Bone Miner Res. 2011. PMID: 21638315
-
A novel therapeutic approach with Caviunin-based isoflavonoid that en routes bone marrow cells to bone formation via BMP2/Wnt-β-catenin signaling.Cell Death Dis. 2014 Sep 18;5(9):e1422. doi: 10.1038/cddis.2014.350. Cell Death Dis. 2014. PMID: 25232676 Free PMC article.
-
Therapeutic potential of phosphodiesterase inhibitors in the treatment of osteoporosis: Scopes for therapeutic repurposing and discovery of new oral osteoanabolic drugs.Eur J Pharmacol. 2021 May 15;899:174015. doi: 10.1016/j.ejphar.2021.174015. Epub 2021 Mar 9. Eur J Pharmacol. 2021. PMID: 33711307 Review.
-
Advances in the roles of ATF4 in osteoporosis.Biomed Pharmacother. 2023 Dec 31;169:115864. doi: 10.1016/j.biopha.2023.115864. Epub 2023 Nov 9. Biomed Pharmacother. 2023. PMID: 37948991 Review.
Cited by
-
Withania somnifera as a Potential Anxiolytic and Anti-inflammatory Candidate Against Systemic Lipopolysaccharide-Induced Neuroinflammation.Neuromolecular Med. 2018 Sep;20(3):343-362. doi: 10.1007/s12017-018-8497-7. Epub 2018 May 30. Neuromolecular Med. 2018. PMID: 29846872
-
FOXO1 regulates RUNX2 ubiquitination through SMURF2 in calcific aortic valve disease.Redox Biol. 2024 Jul;73:103215. doi: 10.1016/j.redox.2024.103215. Epub 2024 May 27. Redox Biol. 2024. PMID: 38810422 Free PMC article.
-
New Emerging Aspect of Herbal Extracts for the Treatment of Osteoporosis: Overview.Curr Rheumatol Rev. 2024;20(4):361-372. doi: 10.2174/0115733971273691231121131455. Curr Rheumatol Rev. 2024. PMID: 38173067 Review.
-
Osteoblast dysfunctions in bone diseases: from cellular and molecular mechanisms to therapeutic strategies.Cell Mol Life Sci. 2015 Apr;72(7):1347-61. doi: 10.1007/s00018-014-1801-2. Epub 2014 Dec 9. Cell Mol Life Sci. 2015. PMID: 25487608 Free PMC article. Review.
-
Comparative transcriptome analysis of different chemotypes elucidates withanolide biosynthesis pathway from medicinal plant Withania somnifera.Sci Rep. 2015 Dec 21;5:18611. doi: 10.1038/srep18611. Sci Rep. 2015. PMID: 26688389 Free PMC article.
References
-
- Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–159. - PubMed
-
- Hie M, Tsukamoto I. Increased expression of the receptor for activation of NF-kappaB and decreased runt-related transcription factor 2 expression in bone of rats with streptozotocin-induced diabetes. Int J Mol Med. 2010;26:611–618. - PubMed
-
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

