Tubular von Hippel-Lindau knockout protects against rhabdomyolysis-induced AKI
- PMID: 23970125
- PMCID: PMC3810090
- DOI: 10.1681/ASN.2013030281
Tubular von Hippel-Lindau knockout protects against rhabdomyolysis-induced AKI
Abstract
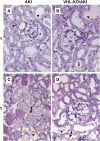
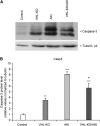
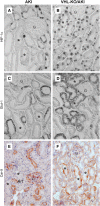
Renal hypoxia occurs in AKI of various etiologies, but adaptation to hypoxia, mediated by hypoxia-inducible factor (HIF), is incomplete in these conditions. Preconditional HIF activation protects against renal ischemia-reperfusion injury, yet the mechanisms involved are largely unknown, and HIF-mediated renoprotection has not been examined in other causes of AKI. Here, we show that selective activation of HIF in renal tubules, through Pax8-rtTA-based inducible knockout of von Hippel-Lindau protein (VHL-KO), protects from rhabdomyolysis-induced AKI. In this model, HIF activation correlated inversely with tubular injury. Specifically, VHL deletion attenuated the increased levels of serum creatinine/urea, caspase-3 protein, and tubular necrosis induced by rhabdomyolysis in wild-type mice. Moreover, HIF activation in nephron segments at risk for injury occurred only in VHL-KO animals. At day 1 after rhabdomyolysis, when tubular injury may be reversible, the HIF-mediated renoprotection in VHL-KO mice was associated with activated glycolysis, cellular glucose uptake and utilization, autophagy, vasodilation, and proton removal, as demonstrated by quantitative PCR, pathway enrichment analysis, and immunohistochemistry. In conclusion, a HIF-mediated shift toward improved energy supply may protect against acute tubular injury in various forms of AKI.
Figures






References
-
- Waikar SS, Liu KD, Chertow GM: Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 - PubMed
-
- Hoste EAJ, Schurgers M: Epidemiology of acute kidney injury: How big is the problem? Crit Care Med 36[Suppl]: S146–S151, 2008 - PubMed
-
- Kellum JA: Acute kidney injury. Crit Care Med 36[Suppl]: S141–S145, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

