Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer
- PMID: 23970364
- PMCID: PMC4404505
- DOI: 10.2967/jnumed.112.113373
Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer
Abstract
The presence of estrogen receptor (ER) in breast cancer is a prognostic indicator for both disease-free and overall survival. 16α-(18)F-fluoro-17β-estradiol ((18)F-FES) with PET is a noninvasive test for evaluation of ER expression and has been used for predicting response to endocrine therapy in patients with ER-positive metastatic breast cancer. The purpose of this study was to correlate (18)F-FES PET and ER expression in patients with primary, operable breast cancer.
Methods: Forty-eight patients were prospectively enrolled in an institutional review board-approved protocol and signed an informed consent form. All patients had undergone (18)F-FES PET preoperatively. Clinical characteristics, tumor characteristics, and treatment outcomes were recorded. Immunohistochemical analysis for ER and progesterone receptor (PgR) percentage expression (46 surgical, 2 core biopsy specimens) was performed. (18)F-FES PET standardized uptake value (SUV) of the breast lesion was correlated with percentage immunohistochemistry ER and PgR expression. (18)F-FES PET SUV was quantified, with a value of 1.5 or more considered positive, and ER and PgR was quantified, with 1% or more considered positive. Formalin-fixed paraffin-embedded tissue was available for 44 patients (42 surgical, 2 core biopsy specimens). We used a microarray platform, and estrogen-related gene expression data (ESR1, ESR2, and PGR) were compared with (18)F-FES PET SUV (Spearman rank correlation). Tumor size, ductal histology, grade, HER2-neu overexpression, PgR expression, estradiol level, body mass index (BMI), and lean BMI were compared with (18)F-FES PET uptake using univariate and multivariate analysis.
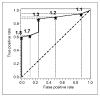
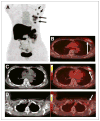
Results: Forty-eight patients completed our protocol, and 2 patients did not undergo surgery because bone metastases were identified preoperatively on (18)F-FES PET. Eighty-three percent of our patients were stage I or II, with a median tumor size of 1.9 cm. Forty-one patients underwent a sentinel node biopsy. Twenty-one patients had nodal involvement. (18)F-FES PET identified 5 patients with axillary nodal uptake (median SUV, 3.0; range, 1.7-6.9). These 5 patients had ER-positive breast cancer, and all had more than 4 positive nodes at the time of axillary node dissection. (18)F-FES PET SUV was associated with immunohistochemistry ER expression. The sensitivity and specificity of the (18)F-FES PET for the breast lesion were 0.85 and 0.75, respectively. Estrogen and progesterone gene expression (ESR1, ESR2, and PGR) was not associated with (18)F-FES PET SUV (Spearman rank correlation). We found a significant correlation between (18)F-FES PET SUV and tumor size (P = 0.0015) but not with ductal histology, grade, HER2-neu overexpression, PgR, estradiol, BMI, or lean BMI (logistic regression). ER expression (P < 0.001) and tumor size (P < 0.0001) were significant on multivariate regression analysis.
Conclusion: (18)F-FES PET SUV correlated with ER immunohistochemistry expression but not gene expression in our patients with early breast cancer. We found that size of primary tumor was significantly associated with (18)F-FES PET SUV. (18)F-FES PET is highly predictive for metastatic disease and helped in the identification of patients with metastatic disease in a preoperative setting.
Keywords: PET imaging; breast cancer; estrogen receptor; ligand.
Figures



References
-
- Fisher ER, Anderson S, Dean S, et al. Solving the dilemma of the immunohistochemical and other methods used for scoring estrogen receptor and progesterone receptor in patients with invasive breast carcinoma. Cancer. 2005;103:164–173. - PubMed
-
- van Netten JP, Armstrong JB, Carlyle SJ, et al. Cellular distribution patterns of estrogen receptor in human breast cancer. Eur J Cancer Clin Oncol. 1988;24:1899–1901. - PubMed
-
- Vollenweider-Zerargui L, Barrelet L, Wong Y, Lemarchand-Beraud T, Gomez F. The predictive value of estrogen and progesterone receptors’ concentrations on the clinical behavior of breast cancer in women: clinical correlation on 547 patients. Cancer. 1986;57:1171–1180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
