Mechanisms and management of diabetic painful distal symmetrical polyneuropathy
- PMID: 23970715
- PMCID: PMC3747929
- DOI: 10.2337/dc12-1964
Mechanisms and management of diabetic painful distal symmetrical polyneuropathy
Abstract
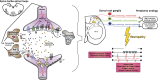
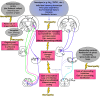
Although a number of the diabetic neuropathies may result in painful symptomatology, this review focuses on the most common: chronic sensorimotor distal symmetrical polyneuropathy (DSPN). It is estimated that 15-20% of diabetic patients may have painful DSPN, but not all of these will require therapy. In practice, the diagnosis of DSPN is a clinical one, whereas for longitudinal studies and clinical trials, quantitative sensory testing and electrophysiological assessment are usually necessary. A number of simple numeric rating scales are available to assess the frequency and severity of neuropathic pain. Although the exact pathophysiological processes that result in diabetic neuropathic pain remain enigmatic, both peripheral and central mechanisms have been implicated, and extend from altered channel function in peripheral nerve through enhanced spinal processing and changes in many higher centers. A number of pharmacological agents have proven efficacy in painful DSPN, but all are prone to side effects, and none impact the underlying pathophysiological abnormalities because they are only symptomatic therapy. The two first-line therapies approved by regulatory authorities for painful neuropathy are duloxetine and pregabalin. α-Lipoic acid, an antioxidant and pathogenic therapy, has evidence of efficacy but is not licensed in the U.S. and several European countries. All patients with DSPN are at increased risk of foot ulceration and require foot care, education, and if possible, regular podiatry assessment.
Figures

Comment in
-
Comment on Tesfaye et al. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes care 2013;36:2456-2465.Diabetes Care. 2014 May;37(5):e120. doi: 10.2337/dc13-3035. Diabetes Care. 2014. PMID: 24757246 No abstract available.
References
-
- Boulton AJM, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care 2004;27:1458–1486 - PubMed
-
- Zelman DC, Brandenburg NA, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain 2006;22:681–685 - PubMed
-
- Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005;30:374–385 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

