Electrostatic control of peptide side-chain reactivity using amphiphilic homopolymer-based supramolecular assemblies
- PMID: 23971726
- PMCID: PMC3836672
- DOI: 10.1021/ja404940s
Electrostatic control of peptide side-chain reactivity using amphiphilic homopolymer-based supramolecular assemblies
Abstract
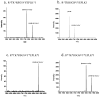
Supramolecular assemblies formed by amphiphilic homopolymers with negatively charged groups in the hydrophilic segment have been designed to enable high labeling selectivity toward reactive side chain functional groups in peptides. The negatively charged interiors of the supramolecular assemblies are found to block the reactivity of protonated amines that would otherwise be reactive in aqueous solution, while maintaining the reactivity of nonprotonated amines. Simple changes to the pH of the assemblies' interiors allow control over the reactivity of different functional groups in a manner that is dependent on the pKa of a given peptide functional group. The labeling studies carried out in positively charged supramolecular assemblies and free buffer solution show that, even when the amine is protonated, labeling selectivity exists only when complementary electrostatic interactions are present, thereby demonstrating the electrostatically controlled nature of these reactions.
Figures





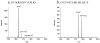


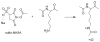
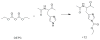

References
-
- Rebek J. Acc Chem Res. 2009;42:1660–1668. - PubMed
- Pluth MD, Bergman RG, Raymond KN. Acc Chem Res. 2009;42:1650–1659. - PubMed
- Yoshizawa M, Klosterman JK, Fujita M. Angew Chem Int Ed. 2009;48:3418–3438. - PubMed
- Vriezema DM, Aragonès MC, Elemans JAAW, Cornelissen JJLM, Rowan AE, Nolte RJM. Chem Rev. 2005;105:1445–1489. - PubMed
- Palivan CG, Onaca OF, Delcea M, Itel F, Meier W. Chem Soc Rev. 2012;41:2800–2823. - PubMed
-
- Liu S, Gan HY, Hermann AT, Rick SW, Gibb BC. Nat Chem. 2010;2:847–852. - PMC - PubMed
- Restorp P, Rebek J. J Am Chem Soc. 2008;130:11850–11851. - PMC - PubMed
- Poul NL, Campion M, Douziech B, Rondelez Y, Clainche LL, Reinaud O, Mest YL. J Am Chem Soc. 2007;129:8801–8810. - PubMed
- Brumaghim JL, Michels M, Raymond KN. Eur J Org Chem. 2004:4552–4559.
- Wang ZJ, Brown CJ, Bergman RG, Raymond KA, Toste FD. J Am Chem Soc. 2011;133:7358–7360. - PubMed
-
- Tascioglu S. Tetrahedron. 1996;52:11113–11152.
- Romsted LS, Bunton CA, Yao J. Curr Opin Coll Interfaces. 1997;2:622–628.
- Holmberg K. Eur J Org Chem. 2007:731–742.
- Oehme G. In: Aqueous-Phase Organometallic Catalysis-Concept and Applications. Cornils B, Herrmann CW, editors. Wiley-VCH; Weinheim: 1998.
- Mandal HK, Majumdar T, Mahapatra A. Int J Chem Kinet. 2011;43:579–589.
- Kaanumalle LS, Nithyanandhan J, Pattabiraman M, Jayaraman N, Ramamurthy V. J Am Chem Soc. 2004;126:8999–9006. - PubMed
- Zayas HA, Lu A, Valade D, Amir F, Jia Z, O’Reilly RK, Monteiro MJ. ACS Macro Lett. 2013;2:327–331. - PubMed
- Lu A, O’Reilly R. Curr Opin Biotech. 2013. Ahead of Print.
- Otto S, Engberts JBFN, Kwak JCT. J Am Chem Soc. 1998;120:9517–9525.
- Klijn JE, Engberts JBFN. J Am Chem Soc. 2003;125:1825–1833. - PubMed
- Wang H, Wang XS, Winnik MA, Manners I. J Am Chem Soc. 2008;130:12921–12930. - PubMed
- Arumugam S, Vutukuri DR, Thayumanavan S, Ramamurthy V. J Am Chem Soc. 2005;127:13200–13206. - PubMed
-
- Tanner P, Onaca O, Balasubramanian V, Meier W, Palivan CG. Chem Eur J. 2011;17:4552–4560. - PubMed
- Yfrach GS, Liddell PA, Hung SC, Moore AL, Gust D, Moore TA. Nature. 1997;385:239–241.
- Stano P, Carrara P, Kuruma Y, Souza TP, Luisi PL. J Mater Chem. 2011;21:18887–18902.
- Smuleac V, Butterfield DA, Bhattacharyya D. Langmuir. 2006;22:10118–10124. - PubMed
- Bi H, Qiao L, Busnel JM, Liu B, Girault HH. J Proteome Res. 2009;8:4685–4692. - PubMed
- Chopineau J, Lesieur S, Taravella BC, Ollivon M. Biochimie. 1998;80:421–435. - PubMed
- Conrado RJ, Varner JD, DeLisa MP. Curr Opin Biotechnol. 2008;19:492–499. - PubMed
- Nardin C, Thoeni S, Widmer J, Winterhalter M, Meier W. Chem Commun. 2000:1433–1434.
-
- Liu CC, Schultz PG. Ann Rev Biochem. 2010;79:413–444. - PubMed
- Young TS, Schultz PG. J Biol Chem. 2010;285:11039–11044. - PMC - PubMed
- Ngo JT, Tirrell DA. Acc Chem Res. 2011;44:677–685. - PMC - PubMed
- Johnson JA, Lu YY, van Deventer JA, Tirrell DA. Curr Opin Chem Biol. 2010;14:774–780. - PMC - PubMed
- Stephanopoulos N, Francis MB. Nat Chem Biol. 2011;7:876–884. - PubMed
- Seim KL, Obermeyer AC, Francis MB. J Am Chem Soc. 2011;133:16970–16976. - PMC - PubMed
- Carrico IS. Chem Soc Rev. 2008;37:1423–1431. - PubMed
- Qi DF, Tann CM, Haring D, Distefano MD. Chem Rev. 2001;101:3081–3111. - PubMed
- Chen I, Howarth M, Lin WY, Ting AY. Nat Methods. 2005;2:99–104. - PubMed
- Hong V, Kislukhin AA, Finn MG. J Am Chem Soc. 2009;131:9986–9994. - PMC - PubMed
- Nonaka H, Fujishima SH, Uchinomiya SH, Ojida A, Hamachi I. J Am Chem Soc. 2010;132:9301–9309. - PubMed
- Antos JM, Chew GL, Guimaraes CP, Yoder NC, Grotenbreg GM, Popp MWL, Ploegh HL. J Am Chem Soc. 2009;131:10800–10181. - PMC - PubMed
- George N, Pick H, Vogel H, Johnsson N, Johnsson K. J Am Chem Soc. 2004;126:8896–8897. - PubMed
- Deuss PJ, Popa G, Botting CH, Laan W, Kamer PCJ. Angew Chem Int Ed. 2010;49:5315–5317. - PubMed
- Ratner V, Kahana E, Eichler M, Haas E. Bioconjugate Chem. 2002;13:1163–1170. - PubMed
- Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. Nat Cell Biol. 2000;2:25–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

