A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation
- PMID: 23971732
- PMCID: PMC4157724
- DOI: 10.2174/15665240113139990076
A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation
Abstract
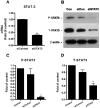
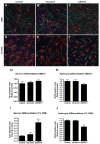
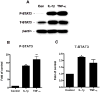
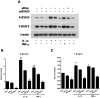
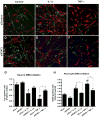
Brain inflammation is a primary pathological driving force of many neurodegenerative disorders. In the destructive process, pro-inflammatory cytokines (IL-1β and TNF-α), are robustly released, affecting normal neural progenitor cell (NPC) differentiation, and resulting in a vast number of astrocytes and a diminished neural population. A counteractive mechanism is still unknown. In this study, we have identified a link between brain inflammation and the signal transducer and activator of transcription 3 (STAT3) pathway: IL-1β and TNF-α induce STAT3 activation in NPCs. Then to investigate STAT3's effects on NPC fate, we observed that an inhibition of STAT3 expression by siRNA inhibited astrocytic differentiation and increased neuronal differentiation of human NPCs in fetal bovine serum (FBS)-induced astrocyte differentiation condition. Furthermore, STAT3-targeting siRNA abrogated IL-1β and TNF-α-induced astrocyte differentiation and partially restored neuronal differentiation. Elimination of STAT3 expression also countered IL-1β and TNF-α-induced inhibition of proneural bHLH genes, mammalian achaete-schute homologue-1 (Mash1), Neurogenin1 (Ngn1), and Neurogenin2 (Ngn2). These data suggest that a suppression of STAT3 during brain inflammation would inhibit astrogliogenesis and promote neurogenesis. Thus, STAT3 could be a potential target of drug therapy for neurodegenerative disorders.
Figures


References
-
- Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8. - PubMed
-
- McKay R. Stem cells in the central nervous system. Science. 1997 Apr 4;276(5309):66–71. - PubMed
-
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. - PubMed
-
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002 Oct 15;202(1-2):13–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
