LOXL2 induces aberrant acinar morphogenesis via ErbB2 signaling
- PMID: 23971878
- PMCID: PMC3978831
- DOI: 10.1186/bcr3461
LOXL2 induces aberrant acinar morphogenesis via ErbB2 signaling
Abstract
Introduction: Lysyl oxidase-like 2 (LOXL2) is a matrix-remodeling enzyme that has been shown to play a key role in invasion and metastasis of breast carcinoma cells. However, very little is known about its role in normal tissue homeostasis. Here, we investigated the effects of LOXL2 expression in normal mammary epithelial cells to gain insight into how LOXL2 mediates cancer progression.
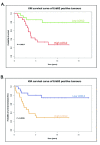
Methods: LOXL2 was expressed in MCF10A normal human mammary epithelial cells. The 3D acinar morphogenesis of these cells was assessed, as well as the ability of the cells to form branching structures on extracellular matrix (ECM)-coated surfaces. Transwell-invasion assays were used to assess the invasive properties of the cells. Clinically relevant inhibitors of ErbB2, lapatinib and Herceptin (traztuzumab), were used to investigate the role of ErbB2 signaling in this model. A retrospective study on a previously published breast cancer patient dataset was carried out by using Disease Specific Genomic Analysis (DSGA) to investigate the correlation of LOXL2 mRNA expression level with metastasis and survival of ErbB2-positive breast cancer patients.
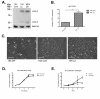
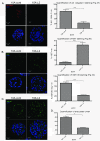
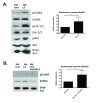
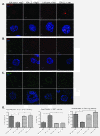
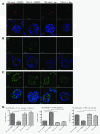
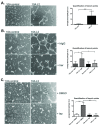
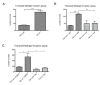
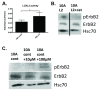
Results: Fluorescence staining of the acini revealed increased proliferation, decreased apoptosis, and disrupted polarity, leading to abnormal lumen formation in response to LOXL2 expression in MCF10A cells. When plated onto ECM, the LOXL2-expressing cells formed branching structures and displayed increased invasion. We noted that LOXL2 induced ErbB2 activation through reactive oxygen species (ROS) production, and ErbB2 inhibition by using Herceptin or lapatinib abrogated the effects of LOXL2 on MCF10A cells. Finally, we found LOXL2 expression to be correlated with decreased overall survival and metastasis-free survival in breast cancer patients with ErbB2-positive tumors.
Conclusions: These findings suggest that LOXL2 expression in normal epithelial cells can induce abnormal changes that resemble oncogenic transformation and cancer progression, and that these effects are driven by LOXL2-mediated activation of ErbB2. LOXL2 may also be a beneficial marker for breast cancer patients that could benefit most from anti-ErbB2 therapy.
Figures
Similar articles
-
Novel mechanism for OSM-promoted extracellular matrix remodeling in breast cancer: LOXL2 upregulation and subsequent ECM alignment.Breast Cancer Res. 2021 May 19;23(1):56. doi: 10.1186/s13058-021-01430-x. Breast Cancer Res. 2021. PMID: 34011405 Free PMC article.
-
Hypoxia/HIF1α induces lapatinib resistance in ERBB2-positive breast cancer cells via regulation of DUSP2.Oncotarget. 2015 Feb 10;6(4):1967-80. doi: 10.18632/oncotarget.2806. Oncotarget. 2015. PMID: 25596742 Free PMC article.
-
Tightly controlled MRTF-A activity regulates epithelial differentiation during formation of mammary acini.Breast Cancer Res. 2017 Jun 7;19(1):68. doi: 10.1186/s13058-017-0860-3. Breast Cancer Res. 2017. PMID: 28592291 Free PMC article.
-
LOXL2 in cancer: regulation, downstream effectors and novel roles.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188435. doi: 10.1016/j.bbcan.2020.188435. Epub 2020 Sep 22. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32976981 Review.
-
Multiple Roles of LOXL2 in the Progression of Hepatocellular Carcinoma and Its Potential for Therapeutic Targeting.Int J Mol Sci. 2023 Jul 21;24(14):11745. doi: 10.3390/ijms241411745. Int J Mol Sci. 2023. PMID: 37511503 Free PMC article. Review.
Cited by
-
LOXL2 in Cancer: A Two-Decade Perspective.Int J Mol Sci. 2023 Sep 21;24(18):14405. doi: 10.3390/ijms241814405. Int J Mol Sci. 2023. PMID: 37762708 Free PMC article. Review.
-
Lysyl oxidase-like 2 inhibitor rescues D-galactose-induced skeletal muscle fibrosis.Aging Cell. 2022 Jul;21(7):e13659. doi: 10.1111/acel.13659. Epub 2022 Jun 17. Aging Cell. 2022. PMID: 35712918 Free PMC article.
-
Human lysyl oxidase-like 2.Bioorg Chem. 2014 Dec;57:231-241. doi: 10.1016/j.bioorg.2014.07.003. Epub 2014 Aug 1. Bioorg Chem. 2014. PMID: 25146937 Free PMC article. Review.
-
LOX overexpression programming mediates the osteoclast mechanism of low peak bone mass in female offspring rats caused by pregnant dexamethasone exposure.Cell Commun Signal. 2023 Apr 24;21(1):84. doi: 10.1186/s12964-023-01115-2. Cell Commun Signal. 2023. PMID: 37095518 Free PMC article.
-
Systematic Characterisation and Analysis of Lysyl Oxidase Family Members as Drivers of Tumour Progression and Multiple Drug Resistance.J Cell Mol Med. 2025 Apr;29(7):e70536. doi: 10.1111/jcmm.70536. J Cell Mol Med. 2025. PMID: 40179101 Free PMC article.
References
-
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;15:1–32. - PubMed
-
- Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;15:1657–1666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

