Molecular characterization of Trichinella spiralis aminopeptidase and its potential as a novel vaccine candidate antigen against trichinellosis in BALB/c mice
- PMID: 23972034
- PMCID: PMC3765106
- DOI: 10.1186/1756-3305-6-246
Molecular characterization of Trichinella spiralis aminopeptidase and its potential as a novel vaccine candidate antigen against trichinellosis in BALB/c mice
Abstract
Background: Trichinella spiralis is an intracellular parasite that can cause a serious threat to human health by causing trichinellosis. The aminopeptidase (AP) was found in the proteins produced by T. spiralis infective larvae after in vitro co-culture with intestinal epithelial cells, but its characteristics and function are unknown. The purpose of this study was to identify the T. spiralis aminopeptidase (TsAP) and to investigate its potential as a vaccine candidate antigen against T. spiralis infection.
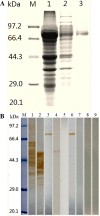
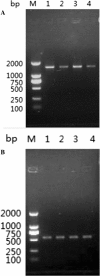
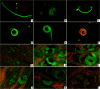
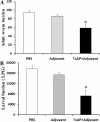
Methods: T. spiralis aminopeptidase (TsAP) gene encoding a 54.7 kDa protein was cloned and expressed in Escherichia coli, and purified recombinant TsAP protein was used to immunize BALB/c mice. The antibodies obtained were used to determine where TsAP was localized in the parasite. Transcription and expression of TsAP in different developmental stages of T. spiralis were observed by RT-PCR and Immunofluorescence test (IFT). The immune protection of recombinant TsAP protein against T. spiralis infection in BALB/c mice was evaluated.
Results: Anti-TsAP antibodies recognized the native protein migrating at 54.7 kDa by Western blotting of the crude antigens from muscle larvae. Transcription and expression of TsAP gene was observed in different developmental stages (adult worms, newborn larvae, pre-encapsulated larvae and muscle larvae). TsAP appears to be a cytoplasmic protein located primarily at the cuticle and internal organs of this parasite. After a challenge infection with T. spiralis infective larvae, mice immunized with the recombinant TsAP protein displayed a 38.1% reduction in adult worm burden and 59.1% reduction in muscle larval burden.
Conclusions: In this study, T. spiralis aminopeptidase (TsAP) was first characterized and will help reveal its potential biological functions. TsAP is a novel potential vaccine candidate antigen that merits further investigation.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

