A randomized trial to optimize HIV/TB care in South Africa: design of the Sizanani trial
- PMID: 23972276
- PMCID: PMC3765953
- DOI: 10.1186/1471-2334-13-390
A randomized trial to optimize HIV/TB care in South Africa: design of the Sizanani trial
Abstract
Background: Despite increases in HIV testing, only a fraction of people newly diagnosed with HIV infection enter the care system and initiate antiretroviral therapy (ART) in South Africa. We report on the design and initial enrollment of a randomized trial of a health system navigator intervention to improve linkage to HIV care and TB treatment completion in Durban, South Africa.
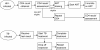
Methods/design: We employed a multi-site randomized controlled trial design. Patients at 4 outpatient sites were enrolled prior to HIV testing. For all HIV-infected participants, routine TB screening with sputum for mycobacterial smear and culture were collected. HIV-infected participants were randomized to receive the health system navigator intervention or usual care. Participants in the navigator arm underwent a baseline interview using a strengths-based case management approach to assist in identifying barriers to entering care and devising solutions to best cope with perceived barriers. Over 4 months, participants in the navigator arm received scheduled phone and text messages. The primary outcome of the study is linkage and retention in care, assessed 9 months after enrollment. For ART-eligible participants without TB, the primary outcome is 3 months on ART as documented in the medical record; participants co-infected with TB are also eligible to meet the primary outcome of completion of 6 months of TB treatment, as documented by the TB clinic. Secondary outcomes include mortality, receipt of CD4 count and TB test results, and repeat CD4 counts for those not ART-eligible at baseline. We hypothesize that a health system navigator can help identify and positively affect modifiable patient factors, including self-efficacy and social support, that in turn can improve linkage to and retention in HIV and TB care.
Discussion: We are currently evaluating the clinical impact of a novel health system navigator intervention to promote entry to and retention in HIV and TB care for people newly diagnosed with HIV. The details of this study protocol will inform clinicians, investigators, and policy makers of strategies to best support HIV-infected patients in resource-limited settings.
Trial registration: Clinicaltrials.gov. unique identifier: NCT01188941.
Figures
References
-
- UNAIDS. HIV and AIDS estimates. 2011. [ http://www.unaids.org/en/regionscountries/countries/southafrica/]
-
- World Health Organization. Global tuberculosis report 2012. http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf.
-
- World Health Organization. Epidemiological country profiles - HIV & AIDS. South Africa; 2008. [ http://www.afro.who.int/en/clusters-a-programmes/dpc/acquired-immune-def...]
-
- Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials