Yield strength of human erythrocyte membranes to impulsive stretching
- PMID: 23972839
- PMCID: PMC3752135
- DOI: 10.1016/j.bpj.2013.06.045
Yield strength of human erythrocyte membranes to impulsive stretching
Abstract
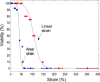
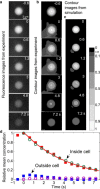
Deformability while remaining viable is an important mechanical property of cells. Red blood cells (RBCs) deform considerably while flowing through small capillaries. The RBC membrane can withstand a finite strain, beyond which it ruptures. The classical yield areal strain of 2-4% for RBCs is generally accepted for a quasi-static strain. It has been noted previously that this threshold strain may be much larger with shorter exposure duration. Here we employ an impulse-like forcing to quantify this yield strain of RBC membranes. In the experiments, RBCs are stretched within tens of microseconds by a strong shear flow generated from a laser-induced cavitation bubble. The deformation of the cells in the strongly confined geometry is captured with a high-speed camera and viability is successively monitored with fluorescence microscopy. We find that the probability of cell survival is strongly dependent on the maximum strain. Above a critical areal strain of ∼40%, permanent membrane damage is observed for 50% of the cells. Interestingly, many of the cells do not rupture immediately and exhibit ghosting, but slowly obtain a round shape before they burst. This observation is explained with structural membrane damage leading to subnanometer-sized pores. The cells finally lyse from the colloidal osmotic pressure imbalance.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Rebuttal to a comment by Richard E. Waugh on our article "Yield strength of human erythrocyte membranes to impulsive stretching".Biophys J. 2014 Apr 15;106(8):1832-3. doi: 10.1016/j.bpj.2014.03.010. Biophys J. 2014. PMID: 24739182 Free PMC article. No abstract available.
-
Forty-percent area strain in red cell membranes?-Doubtful.Biophys J. 2014 Apr 15;106(8):1834-5. doi: 10.1016/j.bpj.2014.01.052. Biophys J. 2014. PMID: 24739183 Free PMC article. No abstract available.
References
-
- Jay A.W.L., Rowlands S., Skibo L. The resistance to blood flow in the capillaries. Can. J. Physiol. Pharmacol. 1972;50:1007–1013. - PubMed
-
- La Celle P.L. Alteration of deformability of the erythrocyte membrane in stored blood. Transfusion. 1969;9:238–245. - PubMed
-
- Schachar N.S., Jay A.W., Tyler F.H. Decreased red cell deformability in burn patients. Can. J. Surg. 1974;17:239–243. - PubMed
-
- Nash G.B., O’Brien E., Dormandy J.A. Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood. 1989;74:855–861. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

