Human PIEZO1: removing inactivation
- PMID: 23972840
- PMCID: PMC3752115
- DOI: 10.1016/j.bpj.2013.07.019
Human PIEZO1: removing inactivation
Abstract
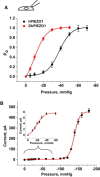
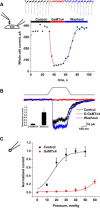
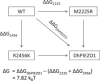
PIEZO1 is an inactivating eukaryotic cation-selective mechanosensitive ion channel. Two sites have been located in the channel that when individually mutated lead to xerocytotic anemia by slowing inactivation. By introducing mutations at two sites, one associated with xerocytosis and the other artificial, we were able to remove inactivation. The double mutant (DhPIEZO1) has a substitution of arginine for methionine (M2225R) and lysine for arginine (R2456K). The loss of inactivation was accompanied by ∼30-mmHg shift of the activation curve to lower pressures and slower rates of deactivation. The slope sensitivity of gating was the same for wild-type and mutants, indicating that the dimensional changes between the closed and open state are unaffected by the mutations. The unitary channel conductance was unchanged by mutations, so these sites are not associated with pore. DhPIEZO1 was reversibly inhibited by the peptide GsMTx4 that acted as a gating modifier. The channel kinetics were solved using complex stimulus waveforms and the data fit to a three-state loop in detailed balance. The reaction had two pressure-dependent rates, closed to open and inactivated to closed. Pressure sensitivity of the opening rate with no sensitivity of the closing rate means that the energy barrier between them is located near the open state. Mutant cycle analysis of inactivation showed that the two sites interacted strongly, even though they are postulated to be on opposite sides of the membrane.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Nilius B., Honoré E. Sensing pressure with ion channels. Trends Neurosci. 2012;35:477–486. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

