Studying kinetochore-fiber ultrastructure using correlative light-electron microscopy
- PMID: 23973081
- PMCID: PMC3805905
- DOI: 10.1016/B978-0-12-407757-7.00020-7
Studying kinetochore-fiber ultrastructure using correlative light-electron microscopy
Abstract
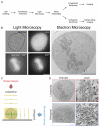
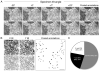
Electron microscopy (EM) has dominated high-resolution cellular imaging for over 50 years, thanks to its ability to resolve on nanometer-scale intracellular structures such as the microtubules of the mitotic spindle. It is advantageous to view the cell of interest prior to processing the sample for EM. Correlative light-electron microscopy (CLEM) is a technique that allows one to visualize cells of interest by light microscopy (LM) before being transferred to EM for ultrastructural examination. Here, we describe how CLEM can be applied as an effective tool to study the spindle apparatus of mitotic cells. This approach allows transfected cells of interest, in desirable stages of mitosis, to be followed from LM to EM. CLEM has often been considered as a technically challenging and laborious technique. In this chapter, we provide step-by-step pictorial guides that allow successful CLEM to be achieved. In addition, we explain how it is possible to vary the sectioning plane, allowing spindles and microtubules to be analyzed from different angles, and the outputs that can be obtained from these methods when applied to the study of kinetochore fiber ultrastructure.
Keywords: Correlative electron microscopy; Kinetochore-fiber; Microtubule; Mitosis; Mitotic spindle.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Engelborghs Y, Heremans KAH, Demaeyer LCM, Hoebeke J. Effect of temperature and pressure on polymerization equilibrium of neuronal microtubules. Nature. 1976;259:686–689. DOI: 10.1038/259686a0. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
