Electrospun scaffolds for tissue engineering of vascular grafts
- PMID: 23973391
- PMCID: PMC3867370
- DOI: 10.1016/j.actbio.2013.08.022
Electrospun scaffolds for tissue engineering of vascular grafts
Abstract
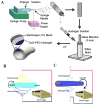
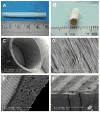
There is a growing demand for off-the-shelf tissue engineered vascular grafts (TEVGs) for the replacement or bypass of damaged arteries in various cardiovascular diseases. Scaffolds from the decellularized tissue skeletons to biopolymers and biodegradable synthetic polymers have been used for fabricating TEVGs. However, several issues have not yet been resolved, which include the inability to mimic the mechanical properties of native tissues, and the ability for long-term patency and growth required for in vivo function. Electrospinning is a popular technique for the production of scaffolds that has the potential to address these issues. However, its application to human TEVGs has not yet been achieved. This review provides an overview of tubular scaffolds that have been prepared by electrospinning with potential for TEVG applications.
Keywords: Electrospinning; Mechanical properties; Tissue engineering; Tubular scaffolds; Vascular grafts.
Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





References
-
- Browning MB, Dempsey D, Guiza V, Becerra S, Rivera J, Russell B, et al. Multilayer vascular grafts based on collagen-mimetic proteins. Acta Biomaterialia. 2011;8:1010–21. - PubMed
-
- Tu JV, Pashos CL, Naylor CD, Chen EL, Normand SL, Newhouse JP, et al. Use of cardiac procedures and outcomes in elderly patients with myocardial infarction in the United States and Canada. New England Journal of Medicine. 1997;336:1500–5. - PubMed
-
- Ratcliffe A. Tissue engineering of vascular grafts. Matrix Biology. 2000;19:353–7. - PubMed
-
- Dolgin E. Taking tissue engineering to heart. Nature Medicine. 2011;17:1032–5. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EB012597/EB/NIBIB NIH HHS/United States
- HL092836/HL/NHLBI NIH HHS/United States
- DE019024/DE/NIDCR NIH HHS/United States
- R01 HL092836/HL/NHLBI NIH HHS/United States
- EB008392/EB/NIBIB NIH HHS/United States
- DE021468/DE/NIDCR NIH HHS/United States
- R01 DE021468/DE/NIDCR NIH HHS/United States
- R01 AR057837/AR/NIAMS NIH HHS/United States
- R01 HL099073/HL/NHLBI NIH HHS/United States
- AR057837/AR/NIAMS NIH HHS/United States
- EB012597/EB/NIBIB NIH HHS/United States
- HL099073S/HL/NHLBI NIH HHS/United States
- RL1 DE019024/DE/NIDCR NIH HHS/United States
- R01 EB008392/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

