Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation
- PMID: 23974041
- PMCID: PMC3891676
- DOI: 10.1038/ncb2828
Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation
Erratum in
- Nat Cell Biol. 2013 Oct;15(10):1260
Abstract
Present models suggest that the fate of the kidney epithelial progenitors is solely regulated by signals from the adjacent ureteric bud. The bud provides signals that regulate the survival, renewal and differentiation of these cells. Recent data suggest that Wnt9b, a ureteric-bud-derived factor, is sufficient for both progenitor cell renewal and differentiation. How the same molecule induces two seemingly contradictory processes is unknown. Here, we show that signals from the stromal fibroblasts cooperate with Wnt9b to promote differentiation of the progenitors. The atypical cadherin Fat4 encodes at least part of this stromal signal. Our data support a model whereby proper kidney size and function is regulated by balancing opposing signals from the ureteric bud and stroma to promote renewal and differentiation of the nephron progenitors.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



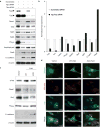



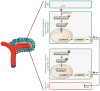
References
-
- Grobstein C. Inductive interaction in the development of the mouse metanephros. J Exp Zool. 1955;130:319–340.
-
- Grobstein C. Inductive epithlio-mesenchymal interaction in cultured organ rudiments of the mouse metanephros. Science. 1953;118:52–55. - PubMed
-
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. - PubMed
-
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

