Gravitational force modulates G2/M phase exit in mechanically unloaded myoblasts
- PMID: 23974110
- PMCID: PMC3875675
- DOI: 10.4161/cc.26029
Gravitational force modulates G2/M phase exit in mechanically unloaded myoblasts
Abstract
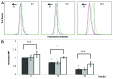
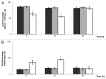
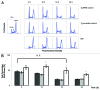
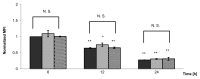
Prolonged spaceflight gives rise to muscle loss and reduced strength, a condition commonly referred to as space atrophy. During exposure to microgravity, skeletal muscle myoblasts are mechanically unloaded and respond with attenuated cell proliferation, slowed cell cycle progression, and modified protein expression. To elucidate the underlying mechanisms by which muscle mass declines in response to prolonged microgravity exposure, we grew C2C12 mouse muscle cells under conditions of simulated microgravity (SM) and analyzed their proliferative capacity, cell cycle progression, and cyclin B and D expression. We demonstrated that the retarded cell growth observed in SM was correlated with an approximate 16 h delay in G2/M phase progression, where cells accumulated specifically between the G2 checkpoint and the onset of anaphase, concomitantly with a positive expression for cyclin B. The effect was specific for gravitational mechanical unloading as cells grown under conditions of hypergravity (HG, 4 g) for similar durations of time exhibited normal proliferation and normal cell cycle progression. Our results show that SM and HG exert phenomenological distinct responses over cell cycle progression. The deficits of SM can be restored by terrestrial gravitational force, whereas the effects of HG are indistinguishable from the 1 g control. This suggests that the mechanotransduction apparatus of cells responds differently to mechanical unloading and loading.
Keywords: cell cycle; cyclins; hypergravity; mechanical unloading; mechanotransduction; microgravity; muscle atrophy.
Figures




Comment in
-
TRPC1: getting physical in space.Cell Cycle. 2013 Nov 1;12(21):3355-6. doi: 10.4161/cc.26637. Epub 2013 Sep 27. Cell Cycle. 2013. PMID: 24091530 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
