Mixed hematopoietic or T-cell chimerism above a minimal threshold restores perforin-dependent immune regulation in perforin-deficient mice
- PMID: 23974195
- PMCID: PMC3795460
- DOI: 10.1182/blood-2013-06-508143
Mixed hematopoietic or T-cell chimerism above a minimal threshold restores perforin-dependent immune regulation in perforin-deficient mice
Abstract
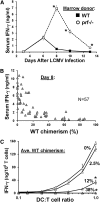
Defects in perforin and related genes lead to a loss of normal immune regulation and underlie hemophagocytic lymphohistiocytosis (HLH), which requires hematopoietic cell transplantation for long-term cure. However, transplantation may be complicated by the development of mixed chimerism and uncertainty regarding the risk of HLH recurrence. To help clarify this risk and investigate how perforin influences immune activation, we studied perforin-mediated immune regulation in the context of mixed chimerism using a murine model of HLH. We found that there is a distinct threshold of ∼10% to 20% perforin expression with either mixed hematopoietic or CD8(+) T cell chimerism, above which immune regulation was reestablished. These findings demonstrate that perforin-mediated immunoregulation functions in trans and are consistent with a feedback model in which cytotoxic T cells control immune activation by killing dendritic cells. These findings also suggest rational targets for maintenance of minimal posttransplant chimerism and for therapeutic strategies involving gene correction.
Figures

References
-
- Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008;42(3):175–180. - PubMed
-
- Cooper N, Rao K, Goulden N, Webb D, Amrolia P, Veys P. The use of reduced-intensity stem cell transplantation in haemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis. Bone Marrow Transplant. 2008;42(Suppl 2):S47–S50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

