Sensitive assay for measurement of volatile borneol, isoborneol, and the metabolite camphor in rat pharmacokinetic study of Borneolum (Bingpian) and Borneolum syntheticum (synthetic Bingpian)
- PMID: 23974515
- PMCID: PMC4002161
- DOI: 10.1038/aps.2013.86
Sensitive assay for measurement of volatile borneol, isoborneol, and the metabolite camphor in rat pharmacokinetic study of Borneolum (Bingpian) and Borneolum syntheticum (synthetic Bingpian)
Abstract
Aim: Both Borneolum (Chinese name Bingpian; dextrorotatory borneol) and Borneolum syntheticum (synthetic Bingpian; a mixture of optically inactive borneol and isoborneol) have been used for medicinal purposes in Chinese traditional medicine. The aim of this study was to develop a sensitive assay for measuring volatile ingredients borneol, isoborneol, and their metabolite camphor in pharmacokinetic study of Bingpian.
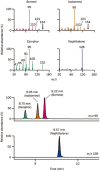
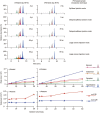
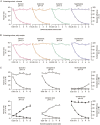
Methods: Rat plasma samples were prepared using liquid-liquid microextraction: 70 μL of plasma sample (containing 125 nmol/L naphthalene as the internal standard) was extracted with 35 μL of n-hexane. The resulting n-hexane extract (20 μL) was introduced into a gas chromatography/mass spectrometry system using programmable temperature vaporizing-based large-volume injection. The assay was validated to demonstrate its reliability for the intended use. Using this assay, pharmacokinetic studies of Bingpian, synthetic Bingpian, and Fufang-Danshen tablets (containing synthetic Bingpian) were conducted in rats.
Results: The extraction efficiency for the analytes and the internal standard from plasma was almost constant with decrease in n-hexane-to-plasma volume ratio, thus enabling a small volume of extracting solvent to be used for sample preparation, and enhancing the assay sensitivity. The lower quantification limit for measuring borneol, isoborneol, and camphor in plasma was 0.98 nmol/L, which was 33-330 times more sensitive than those reported earlier for Bingpian and synthetic Bingpian. The applicability of the miniaturized liquid-liquid extraction technique could be extended to measure other volatile and nonvolatile medicinal compounds in biomatrices, which can be predicted according to the analytes' octanol/water distribution coefficient (logD) and acid dissociation constant (pKa).
Conclusion: This assay is sensitive, accurate and free of matrix effects, and can be applied to pharmacokinetic studies of Bingpian, synthetic Bingpian, and Bingpian-containing herbal products.
Figures


References
-
- Aoshima H, Hamamoto K. Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncid. Biosci Biotechnol Biochem. 1999;63:743–8. - PubMed
-
- Mühlbauer RC, Lozano A, Palacio S, Reinli A, Felix R. Common herbs, essential oils, and monoterpenes potently modulate bone metabolism. Bone. 2003;32:372–80. - PubMed
-
- Huang LS, Gu YF, Li H.Advances in herbal volatile oil and aromatic herbs China J Chin Mater Med 2009341606–11.Chinese. - PubMed
-
- Liang YZ, Xie PS, Chan K. Quality control of herbal medicines. J Chromatogr B. 2004;812:53–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

