Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle
- PMID: 23974906
- PMCID: PMC3828800
- DOI: 10.1161/JAHA.113.000365
Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle
Abstract
Background: KATP channels, assembled from pore-forming (Kir6.1 or Kir6.2) and regulatory (SUR1 or SUR2) subunits, link metabolism to excitability. Loss of Kir6.2 results in hypoglycemia and hyperinsulinemia, whereas loss of Kir6.1 causes Prinzmetal angina-like symptoms in mice. Conversely, overactivity of Kir6.2 induces neonatal diabetes in mice and humans, but consequences of Kir6.1 overactivity are unknown.
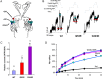
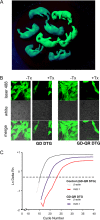
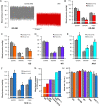
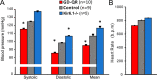
Methods and results: We generated transgenic mice expressing wild-type (WT), ATP-insensitive Kir6.1 [Gly343Asp] (GD), and ATP-insensitive Kir6.1 [Gly343Asp,Gln53Arg] (GD-QR) subunits, under Cre-recombinase control. Expression was induced in smooth muscle cells by crossing with smooth muscle myosin heavy chain promoter-driven tamoxifen-inducible Cre-recombinase (SMMHC-Cre-ER) mice. Three weeks after tamoxifen induction, we assessed blood pressure in anesthetized and conscious animals, as well as contractility of mesenteric artery smooth muscle and KATP currents in isolated mesenteric artery myocytes. Both systolic and diastolic blood pressures were significantly reduced in GD and GD-QR mice but normal in mice expressing the WT transgene and elevated in Kir6.1 knockout mice as well as in mice expressing dominant-negative Kir6.1 [AAA] in smooth muscle. Contractile response of isolated GD-QR mesenteric arteries was blunted relative to WT controls, but nitroprusside relaxation was unaffected. Basal KATP conductance and pinacidil-activated conductance were elevated in GD but not in WT myocytes.
Conclusions: KATP overactivity in vascular muscle can lead directly to reduced vascular contractility and lower blood pressure. We predict that gain of vascular KATP function in humans would lead to a chronic vasodilatory phenotype, as indeed has recently been demonstrated in Cantu syndrome.
Keywords: ABCC9; KATP; KCNJ8; hypotension; mice; transgenic.
Figures


References
-
- Noma A. ATP‐regulated K+ channels in cardiac muscle. Nature. 1983; 305:147-148 - PubMed
-
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP‐sensitive K+ channels in arterial smooth muscle. Science. 1989; 245:177-180 - PubMed
-
- Katnik C, Adams DJ. Characterization of ATP‐sensitive potassium channels in freshly dissociated rabbit aortic endothelial cells. Am J Physiol. 1997; 272:H2507-H2511 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

