Interaction of human serum albumin with novel 3,9-disubstituted perylenes
- PMID: 23975144
- PMCID: PMC3871871
- DOI: 10.1007/s10930-013-9508-z
Interaction of human serum albumin with novel 3,9-disubstituted perylenes
Abstract
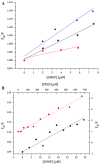
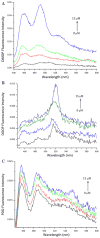
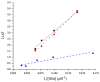
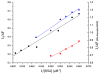
Human serum albumin (HSA) has been used as a model for the binding of a number of different ligands, including polyaromatic hydrocarbons, to proteins. In this case we have investigated the interaction of HSA with a novel set of perylene derivatives. Di-substituted perylene analogues have been synthesized as potentially useful organic photovoltaic materials. Their photophysical properties may make them viable for fuel cell applications too. However, these molecules are poorly soluble especially in aqueous solvents. Binding to water-soluble proteins may provide a way to solubilize them. At the same time one can study whether the photophysical processes initiated by the irradiation of a perylene ligand can cause conformational changes to the host protein. With the present study we demonstrated that of the three perylene derivatives investigated only one, the dimethoxy analogue, has a significant affinity for HSA at a binding site near the bottom of the central cleft (in proximity of the Trp214 residue). The small affinity prevents any significant photoinduced changes to occur in the protein.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

