Assessing the effect of loop mutations in the folding space of β2-microglobulin with molecular dynamics simulations
- PMID: 23975166
- PMCID: PMC3794727
- DOI: 10.3390/ijms140917256
Assessing the effect of loop mutations in the folding space of β2-microglobulin with molecular dynamics simulations
Abstract
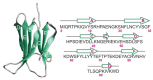
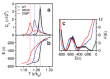
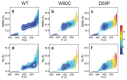
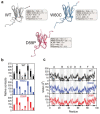
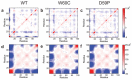
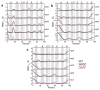
We use molecular dynamics simulations of a full atomistic Gō model to explore the impact of selected DE-loop mutations (D59P and W60C) on the folding space of protein human β2-microglobulin (Hβ2m), the causing agent of dialysis-related amyloidosis, a conformational disorder characterized by the deposition of insoluble amyloid fibrils in the osteoarticular system. Our simulations replicate the effect of mutations on the thermal stability that is observed in experiments in vitro. Furthermore, they predict the population of a partially folded state, with 60% of native internal free energy, which is akin to a molten globule. In the intermediate state, the solvent accessible surface area increases up to 40 times relative to the native state in 38% of the hydrophobic core residues, indicating that the identified species has aggregation potential. The intermediate state preserves the disulfide bond established between residue Cys25 and residue Cys80, which helps maintain the integrity of the core region, and is characterized by having two unstructured termini. The movements of the termini dominate the essential modes of the intermediate state, and exhibit the largest displacements in the D59P mutant, which is the most aggregation prone variant. PROPKA predictions of pKa suggest that the population of the intermediate state may be enhanced at acidic pH explaining the larger amyloidogenic potential observed in vitro at low pH for the WT protein and mutant forms.
Figures
References
-
- Hasegawa K., Ohhashi Y., Yamaguchi I., Takahashi N., Tsutsumi S., Goto Y., Gejyo F., Naiki H. Amyloidogenic synthetic peptides of β2-microglobulin—A role of the disulfide bond. Biochem. Biophys. Res. Commun. 2003;304:101–106. - PubMed
-
- Yamamoto K., Yagi H., Ozawa D., Sasahara K., Naiki H., Goto Y. Thiol compounds inhibit the formation of amyloid fibrils by β2-microglobulin at neutral pH. J. Mol. Biol. 2008;376:258–268. - PubMed
-
- Bellotti V., Gallieni M., Giorgetti S., Brancaccio D. Dynamic of β2-microglobulin fibril formation and reabsorption: The role of proteolysis. Semin. Dial. 2001;14:117–122. - PubMed
-
- Eakin C.M., Miranker A.D. From chance to frequent encounters: Origins of β2-microglobulin fibrillogenesis. BBA-Proteins Proteom. 2005;1753:92–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

