Oxidative stress and microRNAs in vascular diseases
- PMID: 23975169
- PMCID: PMC3794730
- DOI: 10.3390/ijms140917319
Oxidative stress and microRNAs in vascular diseases
Abstract
Oxidative stress has been demonstrated to play a causal role in different vascular diseases, such as hypertension, diabetic vasculopathy, hypercholesterolemia and atherosclerosis. Indeed, increased reactive oxygen species (ROS) production is known to impair endothelial and vascular smooth muscle cell functions, contributing to the development of cardiovascular diseases. MicroRNAs (miRNAs) are non-coding RNA molecules that modulate the stability and/or the translational efficiency of target messenger RNAs. They have been shown to be modulated in most biological processes, including in cellular responses to redox imbalance. In particular, miR-200 family members play a crucial role in oxidative-stress dependent endothelial dysfunction, as well as in cardiovascular complications of diabetes and obesity. In addition, different miRNAs, such as miR-210, have been demonstrated to play a key role in mitochondrial metabolism, therefore modulating ROS production and sensitivity. In this review, we will discuss miRNAs modulated by ROS or involved in ROS production, and implicated in vascular diseases in which redox imbalance has a pathogenetic role.
Figures
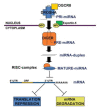

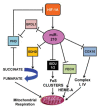
References
-
- Huntzinger E., Izaurralde E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011;12:99–110. - PubMed
-
- Winter J., Jung S., Keller S., Gregory R.I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009;11:228–234. - PubMed
-
- Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. RNA. 2011;3:311–330. - PubMed
-
- Elefant N., Altuvia Y., Margalit H. A wide repertoire of miRNA binding sites: Prediction and functional implications. Bioinformatics. 2011;27:3093–3101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

