STAT3-mediated activation of microRNA cluster 17~92 promotes proliferation and survival of ALK-positive anaplastic large cell lymphoma
- PMID: 23975180
- PMCID: PMC4007939
- DOI: 10.3324/haematol.2013.088286
STAT3-mediated activation of microRNA cluster 17~92 promotes proliferation and survival of ALK-positive anaplastic large cell lymphoma
Abstract
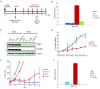
Systemic anaplastic large cell lymphoma is a category of T-cell non-Hodgkin's lymphoma which can be further subdivided into two distinct entities (ALK(+) and ALK(-)) based on the presence or absence of ALK gene rearrangements. Among several pathways triggered by ALK signaling, constitutive activation of STAT3 is strictly required for ALK-mediated transformation and survival. Here we performed genome-wide microRNA profiling and identified 48 microRNA concordantly modulated by the inducible knock-down of ALK and STAT3. To evaluate the functional role of differentially expressed miRNA, we forced their expression in ALK(+) anaplastic large cell lymphoma cells, and monitored their influence after STAT3 depletion. We found that the expression of the microRNA-17~92 cluster partially rescues STAT3 knock-down by sustaining proliferation and survival of ALK(+) cells. Experiments in a xenograft mouse model indicated that forced expression of microRNA-17~92 interferes with STAT3 knock-down in vivo. High expression levels of the microRNA-17~92 cluster resulted in down-regulation of BIM and TGFβRII proteins, suggesting that their targeting might mediate resistance to STAT3 knock-down in anaplastic large cell lymphoma cells. We speculate that the microRNA-17~92 cluster is involved in lymphomagenesis of STAT3(+) ALCL and that its inhibition might represent an alternative avenue to interfere with ALK signaling in anaplastic large cell lymphomas.
Figures



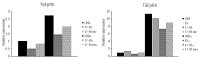
References
-
- Fornari A, Piva R, Chiarle R, Novero D, Inghirami G. Anaplastic large cell lymphoma: one or more entities among T-cell lymphoma¿ Hematol Oncol. 2009;27(4):161–70 - PubMed
-
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30 - PubMed
-
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–4 - PubMed
-
- Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23 - PubMed
-
- Amin HM, Medeiros LJ, Ma Y, Feretzaki M, Das P, Leventaki V, et al. Inhibition of JAK3 induces apoptosis and decreases anaplastic lymphoma kinase activity in anaplastic large cell lymphoma. Oncogene. 2003;22(35):5399–407 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

