Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays
- PMID: 23975197
- PMCID: PMC3834811
- DOI: 10.1093/nar/gkt732
Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays
Abstract
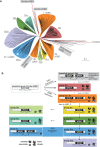
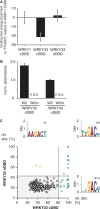
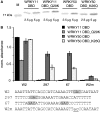
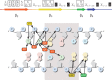
WRKY transcription factors constitute a large protein family in plants that is involved in the regulation of developmental processes and responses to biotic or abiotic stimuli. The question arises how stimulus-specific responses are mediated given that the highly conserved WRKY DNA-binding domain (DBD) exclusively recognizes the 'TTGACY' W-box consensus. We speculated that the W-box consensus might be more degenerate and yet undetected differences in the W-box consensus of WRKYs of different evolutionary descent exist. The phylogenetic analysis of WRKY DBDs suggests that they evolved from an ancestral group IIc-like WRKY early in the eukaryote lineage. A direct descent of group IIc WRKYs supports a monophyletic origin of all other group II and III WRKYs from group I by loss of an N-terminal DBD. Group I WRKYs are of paraphyletic descent and evolved multiple times independently. By homology modeling, molecular dynamics simulations and in vitro DNA-protein interaction-enzyme-linked immunosorbent assay with AtWRKY50 (IIc), AtWRKY33 (I) and AtWRKY11 (IId) DBDs, we revealed differences in DNA-binding specificities. Our data imply that other components are essentially required besides the W-box-specific binding to DNA to facilitate a stimulus-specific WRKY function.
Figures



References
-
- Kilian J, Peschke F, Berendzen KW, Harter K, Wanke D. Prerequisites, performance and profits of transcriptional profiling the abiotic stress response. Biochim. Biophys. Acta. 2012;1819:166–175. - PubMed
-
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

