Modulation of inflammasome-mediated pulmonary immune activation by type I IFNs protects bone marrow homeostasis during systemic responses to Pneumocystis lung infection
- PMID: 23975863
- PMCID: PMC3796383
- DOI: 10.4049/jimmunol.1301344
Modulation of inflammasome-mediated pulmonary immune activation by type I IFNs protects bone marrow homeostasis during systemic responses to Pneumocystis lung infection
Abstract
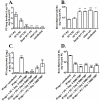
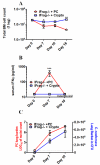
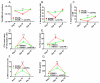
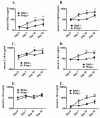
Although acquired bone marrow failure (BMF) is considered a T cell-mediated autoimmune disease, possible innate immune defects as a cause for systemic immune deviations in response to otherwise innocuous infections have not been extensively explored. In this regard, we recently demonstrated an important role of type I IFNs in protecting hematopoiesis during systemic stress responses to the opportunistic fungal pathogen Pneumocystis in lymphocyte-deficient mice. Mice deficient in both lymphocytes and type I IFN receptor (IFrag(-/-) mice) develop rapidly progressing BMF due to accelerated bone marrow (BM) cell apoptosis associated with innate immune deviations in the BM in response to Pneumocystis lung infection. However, the communication pathway between lung and BM eliciting the induction of BMF in response to this strictly pulmonary infection has been unclear. In this study, we report that absence of an intact type I IFN system during Pneumocystis lung infection not only causes BMF in lymphocyte-deficient mice but also transient BM stress in lymphocyte-competent mice. This is associated with an exuberant systemic IFN-γ response. IFN-γ neutralization prevented Pneumocystis lung infection-induced BM depression in type I IFN receptor-deficient mice and prolonged neutrophil survival time in BM from IFrag(-/-) mice. IL-1β and upstream regulators of IFN-γ, IL-12, and IL-18 were also upregulated in lung and serum of IFrag(-/-) mice. In conjunction, there was exuberant inflammasome-mediated caspase-1 activation in pulmonary innate immune cells required for processing of IL-18 and IL-1β. Thus, absence of type I IFN signaling during Pneumocystis lung infection may result in deregulation of inflammasome-mediated pulmonary immune activation, causing systemic immune deviations triggering BMF in this model.
Figures

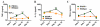
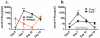
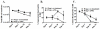
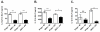
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

