Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase
- PMID: 23975926
- PMCID: PMC3773761
- DOI: 10.1073/pnas.1309648110
Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase
Abstract
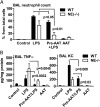
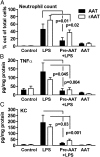
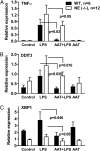
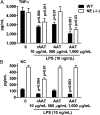
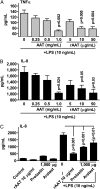
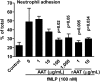
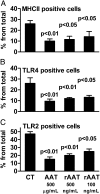
The rationale of α1-antitrypsin (AAT) augmentation therapy to treat progressive emphysema in AAT-deficient patients is based on inhibition of neutrophil elastase; however, the benefit of this treatment remains unclear. Here we show that clinical grade AAT (with elastase inhibitory activity) and a recombinant form of AAT (rAAT) without anti-elastase activity reduces lung inflammatory responses to LPS in elastase-deficient mice. WT and elastase-deficient mice treated with either native AAT or rAAT exhibited significant reductions in infiltrating neutrophils (23% and 68%), lavage fluid levels of TNF-α (70% and 80%), and the neutrophil chemokine KC (CXCL1) (64% and 90%), respectively. Lung parenchyma TNF-α, DNA damage-inducible transcript 3 and X-box binding protein-1 mRNA levels were reduced in both mouse strains treated with AAT; significantly lower levels of these genes, as well as IL-1β gene expression, were observed in lungs of AAT-deficient patients treated with AAT therapy compared with untreated patients. In vitro, LPS-induced cytokines from WT and elastase-deficient mouse neutrophils, as well as neutrophils of healthy humans, were similarly reduced by AAT or rAAT; human neutrophils adhering to endothelial cells were decreased by 60-80% (P < 0.001) with either AAT or rAAT. In mouse pancreatic islet macrophages, LPS-induced surface expression of MHC II, Toll-like receptor-2 and -4 were markedly lower (80%, P < 0.001) when exposed to either AAT or rAAT. Consistently, in vivo and in vitro, rAAT reduced inflammatory responses at concentrations 40- to 100-fold lower than native plasma-derived AAT. These data provide evidence that the anti-inflammatory and immunomodulatory properties of AAT can be independent of elastase inhibition.
Keywords: alpha 1-antitrypsin; immunomodulation; inflammation.
Conflict of interest statement
Conflict of interest statement: S.-H.K., E.C.L., and C.A.D. own stocks in Omni Bio Pharmaceutical.
Figures
References
-
- Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency—A model for conformational diseases. N Engl J Med. 2002;346(1):45–53. - PubMed
-
- Miravitlles M. Alpha-1-antitrypsin and other proteinase inhibitors. Curr Opin Pharmacol. 2012;12(3):309–314. - PubMed
-
- Wewers MD, Crystal RG. Alpha-1 antitrypsin augmentation therapy. COPD. 2013;10(Suppl 1):64–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

