Selective κ opioid antagonists nor-BNI, GNTI and JDTic have low affinities for non-opioid receptors and transporters
- PMID: 23976952
- PMCID: PMC3747596
- DOI: 10.1371/journal.pone.0070701
Selective κ opioid antagonists nor-BNI, GNTI and JDTic have low affinities for non-opioid receptors and transporters
Abstract
Background: Nor-BNI, GNTI and JDTic induce selective κ opioid antagonism that is delayed and extremely prolonged, but some other effects are of rapid onset and brief duration. The transient effects of these compounds differ, suggesting that some of them may be mediated by other targets.
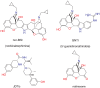
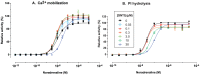
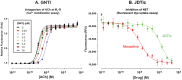
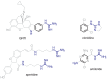
Results: In binding assays, the three antagonists showed no detectable affinity (K(i)≥10 µM) for most non-opioid receptors and transporters (26 of 43 tested). There was no non-opioid target for which all three compounds shared detectable affinity, or for which any two shared sub-micromolar affinity. All three compounds showed low nanomolar affinity for κ opioid receptors, with moderate selectivity over μ and δ (3 to 44-fold). Nor-BNI bound weakly to the α(2C)-adrenoceptor (K(i) = 630 nM). GNTI enhanced calcium mobilization by noradrenaline at the α(1A)-adrenoceptor (EC₅₀ = 41 nM), but did not activate the receptor, displace radioligands, or enhance PI hydrolysis. This suggests that it is a functionally-selective allosteric enhancer. GNTI was also a weak M₁ receptor antagonist (K(B) = 3.7 µM). JDTic bound to the noradrenaline transporter (K(i) = 54 nM), but only weakly inhibited transport (IC₅₀ = 1.1 µM). JDTic also bound to the opioid-like receptor NOP (K(i) = 12 nM), but gave little antagonism even at 30 µM. All three compounds exhibited rapid permeation and active efflux across Caco-2 cell monolayers.
Conclusions: Across 43 non-opioid CNS targets, only GNTI exhibited a potent functional effect (allosteric enhancement of α(1A)-adrenoceptors). This may contribute to GNTI's severe transient effects. Plasma concentrations of nor-BNI and GNTI may be high enough to affect some peripheral non-opioid targets. Nonetheless, κ opioid antagonism persists for weeks or months after these transient effects dissipate. With an adequate pre-administration interval, our results therefore strengthen the evidence that nor-BNI, GNTI and JDTic are highly selective κ opioid antagonists.
Conflict of interest statement
Figures

References
-
- Knoll AT, Carlezon WA Jr (2010) Dynorphin, stress, and depression. Brain Res 1314: 56–73 doi:10.1016/j.brainres.2009.09.074 - DOI - PMC - PubMed
-
- Carlezon WA Jr, Beguin C, Knoll AT, Cohen BM (2009) Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther 123: 334–343 doi:10.1016/j.pharmthera.2009.05.008 - DOI - PMC - PubMed
-
- Béguin C, Cohen BM (2009) Medicinal Chemistry of Kappa Opioid Receptor Antagonists. In: Dean RL, Bilsky EJ, Negus SS, editors. Opiate Receptors and Antagonists: from Bench to Clinic. New York: Humana Press. pp. 99–118. doi:10.1007/978-1-59745-197-0_6
-
- Metcalf M, Coop A (2005) Kappa opioid antagonists: Past successes and future prospects. AAPS J 7: E704–E722 doi:10.1208/aapsj070371 - DOI - PMC - PubMed
-
- Bruchas MR, Yang T, Schreiber S, DeFino M, Kwan SC, et al. (2007) Long-Acting κ Opioid Antagonists Disrupt Receptor Signaling And Produce Noncompetitive Effects By Activating c-Jun N-Terminal Kinase. J Biol Chem 282: 29803–29811 doi:10.1074/jbc.M705540200 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

