Overexpression of the toll-like receptor (TLR) signaling adaptor MYD88, but lack of genetic mutation, in myelodysplastic syndromes
- PMID: 23976989
- PMCID: PMC3744562
- DOI: 10.1371/journal.pone.0071120
Overexpression of the toll-like receptor (TLR) signaling adaptor MYD88, but lack of genetic mutation, in myelodysplastic syndromes
Abstract
MYD88 is a key mediator of Toll-like receptor innate immunity signaling. Oncogenically active MYD88 mutations have recently been reported in lymphoid malignancies, but has not been described in MDS. To characterize MYD88 in MDS, we sequenced the coding region of the MYD88 gene in 40 MDS patients. No MYD88 mutation was detected. We next characterized MYD88 expression in bone marrow CD34+ cells (N = 64). Increased MYD88 RNA was detected in 40% of patients. Patients with higher MYD88 expression in CD34+ cells had a tendency for shorter survival compared to the ones with lower MYD88, which was significant when controlled for IPSS and age. We then evaluated effect of MYD88 blockade in the CD34+ cells of patients with lower-risk MDS. Colony formation assays indicated that MYD88 blockade using a MYD88 inhibitor resulted in increased erythroid colony formation. MYD88 blockade also negatively regulated the secretion of interleukin-8. Treatment of MDS CD34+ cells with an IL-8 antibody also increased formation of erythroid colonies. These results indicate that MYD88 plays a role in the pathobiology of MDS and may have prognostic and therapeutic value in the management of patients with this disease.
Conflict of interest statement
Figures
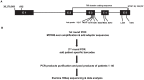
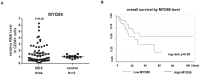

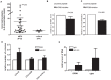
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

