Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption
- PMID: 23977009
- PMCID: PMC3748070
- DOI: 10.1371/journal.pone.0071284
Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption
Abstract
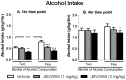
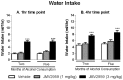
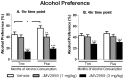
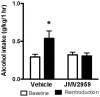
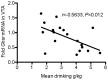
Alcohol dependence is a heterogeneous disorder where several signalling systems play important roles. Recent studies implicate that the gut-brain hormone ghrelin, an orexigenic peptide, is a potential mediator of alcohol related behaviours. Ghrelin increases whereas a ghrelin receptor (GHS-R1A) antagonist decreases alcohol consumption as well as operant self-administration of alcohol in rodents that have consumed alcohol for twelve weeks. In the present study we aimed at investigating the effect of acute and repeated treatment with the GHS-R1A antagonist JMV2959 on alcohol intake in a group of rats following voluntarily alcohol consumption for two, five and eight months. After approximately ten months of voluntary alcohol consumption the expression of the GHS-R1A gene (Ghsr) as well as the degree of methylation of a CpG island found in Ghsr was examined in reward related brain areas. In a separate group of rats, we examined the effect of the JMV2959 on alcohol relapse using the alcohol deprivation paradigm. Acute JMV2959 treatment was found to decrease alcohol intake and the effect was more pronounced after five, compared to two months of alcohol exposure. In addition, repeated JMV2959 treatment decreased alcohol intake without inducing tolerance or rebound increase in alcohol intake after the treatment. The GHS-R1A antagonist prevented the alcohol deprivation effect in rats. There was a significant down-regulation of the Ghsr expression in the ventral tegmental area (VTA) in high- compared to low-alcohol consuming rats after approximately ten months of voluntary alcohol consumption. Further analysis revealed a negative correlation between Ghsr expression in the VTA and alcohol intake. No differences in methylation degree were found between high- compared to low-alcohol consuming rats. These findings support previous studies showing that the ghrelin signalling system may constitute a potential target for development of novel treatment strategies for alcohol dependence.
Conflict of interest statement
Figures


References
-
- Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129. - PubMed
-
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, et al. (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295: 2003–2017. - PubMed
-
- Larsson A, Engel JA (2004) Neurochemical and behavioral studies on ethanol and nicotine interactions. Neuroscience and Biobehavioral Reviews 27: 713–720. - PubMed
-
- Soderpalm B, Lof E, Ericson M (2009) Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry 42 Suppl 1S87–94. - PubMed
-
- Tupala E, Tiihonen J (2004) Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry 28: 1221–1247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

