Rapid cohort generation and analysis of disease spectrum of large animal model of cone dystrophy
- PMID: 23977029
- PMCID: PMC3747164
- DOI: 10.1371/journal.pone.0071363
Rapid cohort generation and analysis of disease spectrum of large animal model of cone dystrophy
Abstract
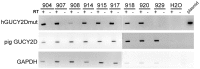
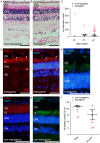
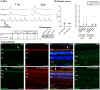
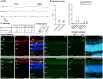
Large animal models are an important resource for the understanding of human disease and for evaluating the applicability of new therapies to human patients. For many diseases, such as cone dystrophy, research effort is hampered by the lack of such models. Lentiviral transgenesis is a methodology broadly applicable to animals from many different species. When conjugated to the expression of a dominant mutant protein, this technology offers an attractive approach to generate new large animal models in a heterogeneous background. We adopted this strategy to mimic the phenotype diversity encounter in humans and generate a cohort of pigs for cone dystrophy by expressing a dominant mutant allele of the guanylate cyclase 2D (GUCY2D) gene. Sixty percent of the piglets were transgenic, with mutant GUCY2D mRNA detected in the retina of all animals tested. Functional impairment of vision was observed among the transgenic pigs at 3 months of age, with a follow-up at 1 year indicating a subsequent slower progression of phenotype. Abnormal retina morphology, notably among the cone photoreceptor cell population, was observed exclusively amongst the transgenic animals. Of particular note, these transgenic animals were characterized by a range in the severity of the phenotype, reflecting the human clinical situation. We demonstrate that a transgenic approach using lentiviral vectors offers a powerful tool for large animal model development. Not only is the efficiency of transgenesis higher than conventional transgenic methodology but this technique also produces a heterogeneous cohort of transgenic animals that mimics the genetic variation encountered in human patients.
Conflict of interest statement
Figures



References
-
- Pacal M, Bremner R (2006) Insights from animal models on the origins and progression of retinoblastoma. Curr Mol Med 6: 759–781. - PubMed
-
- Davidson DJ, Rolfe M (2001) Mouse models of cystic fibrosis. Trends Genet 17: S29–37. - PubMed
-
- Schook L, Beattie C, Beever J, Donovan S, Jamison R, et al. (2005) Swine in biomedical research: creating the building blocks of animal models. Anim Biotechnol 16: 183–190. - PubMed
-
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D (2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295: 868–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/D/05251442/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/05251443/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/07731442/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/05251445/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/05251444/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

