Pyridoxine for prevention of hand-foot syndrome caused by chemotherapy: a systematic review
- PMID: 23977264
- PMCID: PMC3748123
- DOI: 10.1371/journal.pone.0072245
Pyridoxine for prevention of hand-foot syndrome caused by chemotherapy: a systematic review
Abstract
Background: Hand-foot syndrome (HFS) is a relatively frequent dermatologic toxic reaction to certain anti-cancer chemotherapies. The syndrome can evolve into a distressing condition that limits function and affects quality of life. Pyridoxine (vitamin B6) has been used empirically for the prevention of HFS caused by anti-cancer therapy. However, evidence of its efficacy remains controversial.
Methodology//principal findings: Systematic literature searches were conducted on the Cochrane Library, PUBMED, EMBASE, LILACS, CBM, CNKI, VIP, WANFANG and the U.S. ClinicalTrials.gov website. We included all related randomized controlled trials (RCTs) irrespective of language. Reviewers from different professions independently assessed all potential studies and extracted data. Subgroup analysis was planned according to dose of pyridoxine. 5 RCTs involving 607 patients were contributed to the meta-analysis. No significant differences were found between patients receiving pyridoxine and placebo for prevention of incidence of HFS and grade 2 or worse HFS (relative risk (RR) 0.96, 95%confidence interval (CI) 0.86-1.06; RR0.95, 95%CI 0.73-1.24, respectively). Similarly, no significant improvement in quality of life was detected among patients. However, significant difference was found for prevention of grade 2 or worse HFS with pyridoxine 400 mg daily compared to 200 mg (RR0.55, 95%CI 0.33-0.92).
Conclusions/significance: There is inadequate evidence to make any recommendation about using pyridoxine for prevention of HFS caused by chemotherapy. However, pyridoxine 400 mg may have some efficacy. Further studies of large sample sizes are needed to evaluate the efficacy and safety of pyridoxine, especially at high dose, in comparison with placebo.
Conflict of interest statement
Figures




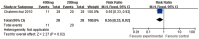
References
-
- Gressett SM, Stanford BL, Hardwicke F (2006) Management of hand-foot syndrome induced by capecitabine. Journal of Oncology Pharmacy Practice 12: 131–141. - PubMed
-
- Nagore E, Insa A, Sanmartín O (2000) Antineoplastic Therapy-Induced Palmar Plantar Erythrodysesthesia ('Hand-Foot') Syndrome: Incidence, Recognition and Management. American Journal of Clinical Dermatology 1: 225–234. - PubMed
-
- Lipworth AD, Robert C, Zhu AX (2009) Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology 77: 257–271. - PubMed
-
- von Moos R, Thuerlimann BJ, Aapro M, Rayson D, Harrold K, et al. (2008) Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer 44: 781–790. - PubMed
-
- Degen A, Alter M, Schenck F, Satzger I, Völker B, et al. (2010) The hand-foot-syndrome associated with medical tumor therapy–classification and management. Journal der Deutschen Dermatologischen Gesellschaft 8: 652–661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

