Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy
- PMID: 23977276
- PMCID: PMC3747157
- DOI: 10.1371/journal.pone.0072301
Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy
Abstract
Progressive aggregation of protein Tau into oligomers and fibrils correlates with cognitive decline and synaptic dysfunction, leading to neurodegeneration in vulnerable brain regions in Alzheimer's disease. The unmet need of effective therapy for Alzheimer's disease, combined with problematic pharmacological approaches, led the field to explore immunotherapy, first against amyloid peptides and recently against protein Tau. Here we adapted the liposome-based amyloid vaccine that proved safe and efficacious, and incorporated a synthetic phosphorylated peptide to mimic the important phospho-epitope of protein Tau at residues pS396/pS404. We demonstrate that the liposome-based vaccine elicited, rapidly and robustly, specific antisera in wild-type mice and in Tau.P301L mice. Long-term vaccination proved to be safe, because it improved the clinical condition and reduced indices of tauopathy in the brain of the Tau.P301L mice, while no signs of neuro-inflammation or other adverse neurological effects were observed. The data corroborate the hypothesis that liposomes carrying phosphorylated peptides of protein Tau have considerable potential as safe and effective treatment against tauopathies, including Alzheimer's disease.
Conflict of interest statement
Figures
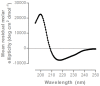
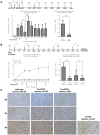
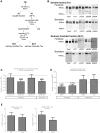

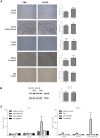
References
-
- Cleveland DW, Hwo SY, Kirschner MW (1977) Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol 2: 227–247. - PubMed
-
- Chen J, Kanai Y, Cowan NJ, Hirokawa N (1992) Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature 6405: 674–677. - PubMed
-
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, et al. (1993) Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 32: 24374–24384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

