Intestinal-specific TNFα overexpression induces Crohn's-like ileitis in mice
- PMID: 23977323
- PMCID: PMC3748077
- DOI: 10.1371/journal.pone.0072594
Intestinal-specific TNFα overexpression induces Crohn's-like ileitis in mice
Abstract
Background and aim: Human and animal studies have clearly established tumor necrosis factor (TNF)α as an important mediator of Crohn's disease pathogenesis. However, whether systemic or only local TNFα overproduction is required for the development of chronic intestinal inflammation and Crohn's disease remains unclear. The aim of this study was to assess the contribution of intestinal epithelial-derived TNFα to the development of murine Crohn's-like ileitis.
Methods: We adapted the well-established TNF(∆ARE/+) mouse model of Crohn's disease (which systemically overexpresses TNFα) to generate a homozygous mutant strain that overexpress TNFα only within the intestinal epithelium. Intestinal-specific TNF(i∆ARE/i∆ARE) mice were examined for histopathological signs of gut inflammation and extraintestinal manifestations of Crohn's disease. The mucosal immune phenotype was characterized, and the contribution of specific lymphocyte populations to the pathogenesis of TNF(i∆ARE/i∆ARE) ileitis was assessed.
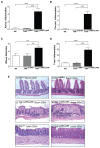
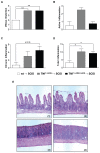
Results: TNF(i∆ARE/i∆ARE) mice had increased mucosal and systemic TNFα levels compared to wild-type controls (P<0.001), as well as severe chronic ileitis with increased neutrophil infiltration and villous distortion, but no extraintestinal manifestations (P<0.001 vs. wild-type controls). The gut mucosal lymphocytic compartment was also expanded in TNF(i∆ARE/i∆ARE) mice (P<0.05), consisting of activated CD69(+) and CD4(+)CD62L(-) lymphocytes (P<0.05). FasL expression was significantly elevated in the mesenteric lymph nodes of TNF(i∆ARE/i∆ARE) mice (P<0.05). Adoptive transfer of mucosal TNF(i∆ARE/i∆ARE) lymphocytes resulted in ileitis in immunologically naïve severe combined immunodeficiency recipients (P<0.05 vs. wild-type controls), indicating an effector phenotype that was associated with increased production of both Th1 (IFNγ) and Th2 (IL-5, IL-13) cytokines.
Conclusion: Intestinal epithelial-derived TNFα is sufficient for the induction of Crohn's-like ileitis, but not for the occurrence of extraintestinal manifestations, in TNF(i∆ARE/i∆ARE) mice. These effects were associated with generation of effector lymphocytes within the intestinal mucosa and dysregulated apoptosis. Thus, targeted intestinal blockade of TNFα may provide an effective means to neutralize gut-derived TNFα with reduced side effects.
Conflict of interest statement
Figures
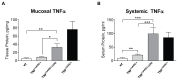


References
-
- Bamias G, Nyce MR, De La Rue SA, Cominelli F, American College of P, et al. (2005) New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med 143: 895-904. - PubMed
-
- Papadakis KA, Targan SR (2000) Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51: 289-298. doi:10.1146/annurev.med.51.1.289. PubMed: 10774465. - DOI - PubMed
-
- Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104: 487-501. doi:10.1016/S0092-8674(01)00237-9. PubMed: 11239407. - DOI - PubMed
-
- Matsuda R, Koide T, Tokoro C, Yamamoto T, Godai T et al. (2009) Quantitative cytokine mRNA expression profiles in the colonic mucosa of patients with steroid naive ulcerative colitis during active and quiescent disease. Inflamm Bowel Dis 15: 328-334. doi:10.1002/ibd.20759. PubMed: 18942752. - DOI - PubMed
-
- Raddatz D, Bockemühl M, Ramadori G (2005) Quantitative measurement of cytokine mRNA in inflammatory bowel disease: relation to clinical and endoscopic activity and outcome. Eur J Gastroenterol Hepatol 17: 547-557. doi:10.1097/00042737-200505000-00012. PubMed: 15827446. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

