Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats
- PMID: 23977328
- PMCID: PMC3747086
- DOI: 10.1371/journal.pone.0072610
Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats
Abstract
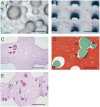
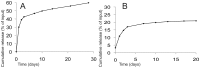
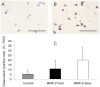
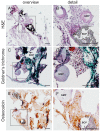
The design of bioactive three-dimensional (3D) scaffolds is a major focus in bone tissue engineering. Incorporation of growth factors into bioprinted scaffolds offers many new possibilities regarding both biological and architectural properties of the scaffolds. This study investigates whether the sustained release of bone morphogenetic protein 2 (BMP-2) influences osteogenicity of tissue engineered bioprinted constructs. BMP-2 loaded on gelatin microparticles (GMPs) was used as a sustained release system, which was dispersed in hydrogel-based constructs and compared to direct inclusion of BMP-2 in alginate or control GMPs. The constructs were supplemented with goat multipotent stromal cells (gMSCs) and biphasic calcium phosphate to study osteogenic differentiation and bone formation respectively. BMP-2 release kinetics and bioactivity showed continuous release for three weeks coinciding with osteogenicity. Osteogenic differentiation and bone formation of bioprinted GMP containing constructs were investigated after subcutaneous implantation in mice or rats. BMP-2 significantly increased bone formation, which was not influenced by the release timing. We showed that 3D printing of controlled release particles is feasible and that the released BMP-2 directs osteogenic differentiation in vitro and in vivo.
Conflict of interest statement
Figures

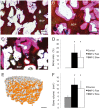
References
-
- Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: an update. Injury 36 Suppl 3S20–27. - PubMed
-
- Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, et al. (2003) Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 28: 134–139. - PubMed
-
- Cross M, Dexter TM (1991) Growth factors in development, transformation, and tumorigenesis. Cell 64: 271–280. - PubMed
-
- Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I (1994) Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol 161: 218–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

