Theta burst stimulation applied over primary motor and somatosensory cortices produces analgesia unrelated to the changes in nociceptive event-related potentials
- PMID: 23977382
- PMCID: PMC3748010
- DOI: 10.1371/journal.pone.0073263
Theta burst stimulation applied over primary motor and somatosensory cortices produces analgesia unrelated to the changes in nociceptive event-related potentials
Abstract
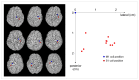
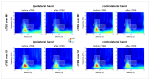
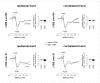
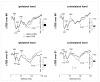
Continuous theta burst stimulation (cTBS) applied over the primary motor cortex (M1) can alleviate pain although the neural basis of this effect remains largely unknown. Besides, the primary somatosensory cortex (S1) is thought to play a pivotal role in the sensori-discriminative aspects of pain perception but the analgesic effect of cTBS applied over S1 remains controversial. To investigate cTBS-induced analgesia we characterized, in two separate experiments, the effect of cTBS applied either over M1 or S1 on the event-related brain potentials (ERPs) and perception elicited by nociceptive (CO2 laser stimulation) and non-nociceptive (transcutaneous electrical stimulation) somatosensory stimuli. All stimuli were delivered to the ipsilateral and contralateral hand. We found that both cTBS applied over M1 and cTBS applied over S1 significantly reduced the percept elicited by nociceptive stimuli delivered to the contralateral hand as compared to similar stimulation of the ipsilateral hand. In contrast, cTBS did not modulate the perception of non-nociceptive stimuli. Surprisingly, this side-dependent analgesic effect of cTBS was not reflected in the amplitude modulation of nociceptive ERPs. Indeed, both nociceptive (N160, N240 and P360 waves) and late-latency non-nociceptive (N140 and P200 waves) ERPs elicited by stimulation of the contralateral and ipsilateral hands were similarly reduced after cTBS, suggesting an unspecific effect, possibly due to habituation or reduced alertness. In conclusion, cTBS applied over M1 and S1 reduces similarly the perception of nociceptive inputs originating from the contralateral hand, but this analgesic effect is not reflected in the magnitude of nociceptive ERPs.
Conflict of interest statement
Figures



Similar articles
-
Deep continuous theta burst stimulation of the operculo-insular cortex selectively affects Aδ-fibre heat pain.J Physiol. 2018 Oct;596(19):4767-4787. doi: 10.1113/JP276359. Epub 2018 Sep 4. J Physiol. 2018. PMID: 30085357 Free PMC article.
-
Human primary somatosensory cortex is differentially involved in vibrotaction and nociception.J Neurophysiol. 2017 Jul 1;118(1):317-330. doi: 10.1152/jn.00615.2016. Epub 2017 Apr 26. J Neurophysiol. 2017. PMID: 28446584 Free PMC article.
-
MEP Latencies Predict the Neuromodulatory Effect of cTBS Delivered to the Ipsilateral and Contralateral Sensorimotor Cortex.PLoS One. 2015 Aug 11;10(8):e0133893. doi: 10.1371/journal.pone.0133893. eCollection 2015. PLoS One. 2015. PMID: 26263505 Free PMC article.
-
Effects of continuous theta-burst stimulation of the primary motor and secondary somatosensory areas on the central processing and the perception of trigeminal nociceptive input in healthy volunteers.Pain. 2019 Jan;160(1):172-186. doi: 10.1097/j.pain.0000000000001393. Pain. 2019. PMID: 30204647 Free PMC article.
-
Effects of transcranial theta-burst stimulation on acute pain perception.Restor Neurol Neurosci. 2010;28(4):477-84. doi: 10.3233/RNN-2010-0555. Restor Neurol Neurosci. 2010. PMID: 20714072 Review.
Cited by
-
Prolonged Continuous Theta Burst Stimulation of the Motor Cortex Modulates Cortical Excitability But not Pain Perception.Front Syst Neurosci. 2020 May 29;14:27. doi: 10.3389/fnsys.2020.00027. eCollection 2020. Front Syst Neurosci. 2020. PMID: 32670027 Free PMC article.
-
Network Effects of Brain Lesions Causing Central Poststroke Pain.Ann Neurol. 2022 Nov;92(5):834-845. doi: 10.1002/ana.26468. Epub 2022 Aug 17. Ann Neurol. 2022. PMID: 36271755 Free PMC article.
-
Designing Brains for Pain: Human to Mollusc.Front Physiol. 2018 Aug 2;9:1027. doi: 10.3389/fphys.2018.01027. eCollection 2018. Front Physiol. 2018. PMID: 30127750 Free PMC article.
-
Does Motor Cortex Engagement During Movement Preparation Differentially Inhibit Nociceptive Processing in Patients with Chronic Whiplash Associated Disorders, Chronic Fatigue Syndrome and Healthy Controls? An Experimental Study.J Clin Med. 2020 May 18;9(5):1520. doi: 10.3390/jcm9051520. J Clin Med. 2020. PMID: 32443565 Free PMC article.
-
Deep continuous theta burst stimulation of the operculo-insular cortex selectively affects Aδ-fibre heat pain.J Physiol. 2018 Oct;596(19):4767-4787. doi: 10.1113/JP276359. Epub 2018 Sep 4. J Physiol. 2018. PMID: 30085357 Free PMC article.
References
-
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC (2005) Theta burst stimulation of the human motor cortex. Neuron 45: 201-206. doi:10.1016/j.neuron.2004.12.033. PubMed: 15664172. - DOI - PubMed
-
- Poreisz C, Csifcsák G, Antal A, Levold M, Hillers F et al. (2008) Theta burst stimulation of the motor cortex reduces laser-evoked pain perception. Neuroreport 19: 193-196. doi:10.1097/WNR.0b013e3282f45498. PubMed: 18185107. - DOI - PubMed
-
- Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG et al. (2012) Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain 16: 1403-1413. doi:10.1002/j.1532-2149.2012.00150.x. PubMed: 22508405. - DOI - PubMed
-
- Melzack R, Casey K (1968) Sensory, motivational, and central control determinants of pain: a new conceptual model. The skin senses Kenshalo R. Springfield: pp. 423–443.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

